#cystitis etiology
Text
Establishment and validation of a rodent model of endometriosis to evaluate the effect of new therapeutic strategies
Endometriosis is the presence of ectopic endometrial tissue outside the uterine cavity and is a common gynecological disease reported in 10% - 15% of women during their reproductive years in the world [1]. The most common symptom of endometriosis is debilitating pelvic/abdominal pain. In fact, up to 70% of women suffering from pelvic pain are affected by endometriosis. Other symptoms include dyspareunia, severe dysmenorrhea, and dysuria. Women with the condition also suffer from co-occurring painful conditions, including interstitial cystitis/painful bladder symptoms and irritable bowel syndrome and 50% of these women suffer from infertility [2].
The disease has a huge negative impact on women’s quality of life, work productivity, sexual relationship and self-esteem [3]. The most common theory proposed to explain the pathophysiology of the endometriosis is retrograde menstruation hypothesis but also other hypotheses have been discussed like inflammatory factors, dysregulated immunity, hormones and genetic and epigenetic factors [4]. None of these mechanisms could explain alone the different types and symptoms of endometriosis. Current therapeutic options have limited insight into the disease mechanisms and include drugs and/or surgery to reduce symptoms and manage complications. These available treatments provide short-term solutions but tend to be ineffective over the long term with a high incidence of unwanted side effects such as premature bone loss and vaginal dryness. The most common medical treatment for endometriosis is gonadotropin-releasing hormone (GnRH) agonists and estrogen/progestin combinations [5,6]. In recent years, the search for more effective and less invasive therapeutic curative strategies to reduce years of suffering in women with endometriosis is receiving increased research attention. The development and validation of In vivo models are necessary to allow the preclinical testing of the efficacy of potential new treatment options.
Non-human primates are considered the most physiologically relevant model of endometriosis since these animals develop spontaneous endometriosis [7]. However, their use is limited by cost and ethical concerns. Thus, rodent models of endometriosis [8] have been developed to investigate the effect of the presence of endometriosis lesions on functional outcomes and the extent of its reversal by new compounds. However, it is difficult to develop a unique model that replicates all symptoms and aspects of a complex disease such as endometriosis. Indeed, endometriosis is a heterogeneous disease with poorly known etiology and several phenotypes such as ovarian endometrioma, superficial peritoneal disease and deep infiltrating endometriosis [4]. In addition, endometriosis is multifactorial and has been associated with environmental, genetic, immunological and hormonal factors [9]. Different models have been developed in mice and rats and recently reviewed by Bruner-Tran, et al. [8], each model emphasizing one or several features to explore disease-related mechanisms, to identify therapeutic targets and/or to provide insight into the development of co-morbidities. Pain is considered a significant contributor to endometriosis morbidity and pelvic pain is one of the most described symptoms [10], even though asymptomatic cases are described [11], this study aimed at establishing a rodent model of endometriosis to be a useful tool in evaluating endometriosis-related pelvic pain. To that end, we developed a rodent model of endometriosis in immunocompetent rats based on surgically-induced endometriosis originally described by Vermon and Wilson [12] to evaluate endometriosis-induced pain by assessing specifically pelvic pain while others extensively evaluated generalized pain behavior. In addition, endometriosis diagnosis is based on the analysis of the lesions collected during laparoscopic surgery [13] and the American Society of Reproductive Medicine classified the disease into 4 stages according to the evaluation of the endometriotic lesions [14]. Thus, this study also described the proliferation of endometriosis lesions being a major feature of endometriosis. Even though the model described in this study is based on previously published studies reviewed by others [8,15], very few authors reported the impact of the estrous stage on endometriosis-induced models in rodents. Hence, this study was designed to investigate if any influence of the estrous stage at induction of endometriosis on both primary endpoints i.e., pelvic pain and endometriosis lesions size. To validate the translational value of this model, we tested the effect of one of the most clinically used medical options, a GnRH agonist on pelvic pain and lesions size.
Materials and methodsEndometriosis-induced surgery and treatment administration
Adult non-pregnant female Sprague Dawley rats (Elevage Janvier, Le Genest-St-Isle, France, 6-8 weeks old) were housed at least 10 days prior to the beginning of the experiments with free access to standard chow (Chow M20, 841201, SDS, UK) and water and maintained on an inversed 12h dark/light cycle (10:00/22:00). All procedures were approved by the local ethical committee (CEE47) and performed in accordance with the legislation on the use of laboratory animals (NIH publication N° 85 - 23, revised 1996) and Animal Care Regulations in force in France as of 1988 (authorization from competent French Ministry of Agriculture - Agreement No. B78-423-1, July 2017).
https://www.peertechzpublications.com/articles/JGRO-8-214.php
0 notes
Text
INFECTION OF THE URINARY TRACT: CAUSES, COMPLICATIONS, AND TREATMENT

An infection of the kidneys, ureters, bladder, or urethra is known as a urinary tract infection (UTI). Most infections affect the lower urinary system, which includes the bladder and urethra. Compared to men, women are more likely to acquire a UTI.
A urinary tract infection affects the kidney, ureter, bladder, and urethra and is characterised by infection. The lower urinary tract, which includes the bladder and urethra, is where the majority of infections occur. Depending on the site in question, various terminology is frequently used, including:
Cystitis: Is an infection that only affects the bladder. Burning micturition, frequent or urgent urination, and/or suprapubic pain are the typical symptoms.
Pyelonephritis: Kidney infection called pyelonephritis. It is the most serious type of UTI and manifests as nausea with or without vomiting, flank pain, fever with chills or rigours, and nausea.
Urethritis: Infection of the urethra is referred to as urethritis. Burning discomfort while urinating and an unpleasant-smelling urethral discharge are typical symptoms.
The following terms are crucial when discussing urinary tract infections:
Complicated UTI: All men, pregnant women, patients with anatomical or functional abnormalities of the urinary tract, those with indwelling urinary catheters, renal illnesses, and/or other immunocompromised conditions are considered to have difficult UTIs.
Bacteria: E. coli, Klebsiella, Proteus, Pseudomonas, Enterococcus, and Staphylococcus are prevalent bacteria.
Fungal: Candida species
Etiology: investigating or assigning a cause or reason for something, frequently using a historical or mythical justification.
Tubercular: constituting or affected with tuberculosis a tuberculosis process.
Symptoms and signs
Urgency is a recurring need to urinate.
Dysuria is the condition in which urination burns.
Passing pee frequently and in little volumes.
turbid, cloudy urine
Urine that is red, bright pink, or cola-collared indicates that there is blood in it.
urine with a foul odour
Suprapubic, bilateral flanks, pelvic discomfort in women, and perineal pain in the abdomen
Risk components
Postmenopausal, sexually active women
Age > 60, Diabetes Mellitus, Post-Transplant, Immunosuppressive Drug Use, and Immunocompromised State
Vesicoureteral reflux, neurogenic bladder, posterior urethral valve, calculus in the urinary tract, urethral stricture, etc. are structural or functional disorders of the urinary tract.
Pregnancy
Instrumentation for the urinary tract
The diagnosis is made using the following tests: imaging tests such as ultrasound and CT scan, history, physical examination, urine routine, and culture sensitivity.
Treatment:
Using the proper antibiotics and antifungals (according to culture reports)
If you have a severe, complicated UTI, you should use IV antibiotics (associated with septicaemia, shock, acute kidney injury)
Surgical removal of stones and correction of urethral stenosis are examples of definitive treatment for structural or functional abnormalities of the urinary tract.
Oral antibiotic prophylaxis for up to three months in the event of recurrent UTI
0 notes
Text
A Novel Strategy for the treatment of Cancer: MCL1 Inhibitors as well as DNA
In a some other bunch within the mind, found in-between the particular optic lobe along with the antennal lobe, many of us recognized much more nerves within In. vitripennis weighed against D. giraulti. Mixing our final results with findings created in the past throughout other Hymenopteran varieties, we #Link# focus on possible functions and a few of the best aspects influencing the actual development with the octopaminergic program in the bug human brain.Purpose: Interstitial cystitis is really a very prevalent pain issue projected to be able to have an effect on 3% in order to 6% of ladies in the usa. Emerging info propose you'll find main neurobiological parts to the etiology of the illness. Many of us document the initial mental faculties architectural photo findings from your MAPP network along with data upon more than 3 hundred participants. Supplies and techniques: All of us used voxel primarily based morphometry to ascertain whether or not man sufferers together with continual interstitial cystitis show adjustments to brain #Link# morphology in comparison with healthy regulates. A total of Thirty three woman patients together with interstitial cystitis with no comorbidities and Thirty-three get older and girl or boy harmonized settings obtained from the more expensive taste went through constitutionnel permanent magnet resonance photo at Your five MAPP sites throughout the U . s .. Benefits: Compared to settings, girls with interstitial cystitis exhibited significant improved grey make any difference amount in a number of regions of the mind including the proper major somatosensory cortex, the superior parietal lobule bilaterally and also the proper extra generator region. Dreary make a difference size within the proper #Link# main somatosensory cortex has been linked to greater soreness, mood (anxiousness) along with urological signs and symptoms. Many of us explored these kind of correlations in a linear regression style, determined independent connection between these kind of Three or more procedures in primary somatosensory cortex grey make any difference size, specifically specialized medical discomfort (McGill discomfort nerve organs overall), a stride regarding urgency as well as anxiety (HADS). Conclusions: These kind of info secure the belief in which adjustments to somatosensory dull make a difference may have an important role experiencing pain level of responsiveness in addition to effective and also sensory areas of interstitial cystitis. Additional studies are had to read the generalizability of these conclusions along with other soreness conditions.Put together coagulation factor VII (FVII) and issue A (Forex) lack (combined FVII/FX deficit) is among the number of blood loss issues by which the two elements show reduced plasma televisions activity. It may well happen coming from coincidental monetary gift of distinct coagulation aspect deficiencies or perhaps a common lead to while huge deletions containing the two gene loci. The F7 along with F10 genetics are placed for the lengthy arm regarding chromosome 12. The following, many of us identify 15 instances using blended FVII/FX deficiency symbolizing both hereditary systems associated with occurrence. Anatomical studies included primary sequencing with the F7 along with F10 genes as well as MLPA (multiplex ligation-dependent probe audio) with regard to diagnosis of heterozygous large deletions. Throughout four individuals, the particular mixed deficit has been as a result of huge removal inside terminal conclusion associated with chromosome 12.
0 notes
Photo

Patreon | Ko-fi
#studyblr#notes#medblr#medical notes#med notes#cystitis#etiology#cystitis etiology#causes of cystitis#anatomy and physiology#anatomy#physiology#pathophysiology#pathology#pathology notes#pathophysiology notes#cystitis causes
6 notes
·
View notes
Text
As an orphan disease interstitial cystitis/bladder pain syndrome (IC/BPS) is a frequently underdiagnosed and inadequately treated disease of the urinary bladder, often after years of symptoms. Caused by an unknown etiology, a high variability of symptoms, a lack of biomarkers and a gradual onset, IC/BPS is a diagnosis by exclusion and poses a special challenge to doctors and patients. In addition to conventional and complementary medical treatment, oral medication, intravesical and transurethral procedures are available as treatment options. Due to the invasiveness or irreversibility, however, interventional surgical procedures should only be used after careful consideration or as a last resort. In order to find a suitable individualized treatment, a classification of the patients according to the severity and type of symptoms can be advantageous.
As mentioned: The precise etiology of BPS is not fully understood. Chronic bacterial infection, defective glycosaminoglycan (GAG) layer of the bladder urothelium, inappropriate activation of mast cells in the suburothelial layer of the bladder, autoimmune-mediated mechanisms and autonomic nervous system dysfunction have all been implicated. Treatments targeted at each of these mechanisms have been developed with mixed outcomes. High-quality research into the treatment options is lacking and it is difficult to draw definite conclusions. The treatment approach is multimodal and should be patient specific, targeting the symptoms which they find most bothersome... but we are at a time in medicine where presently, the treatments at hand are simple not enough.
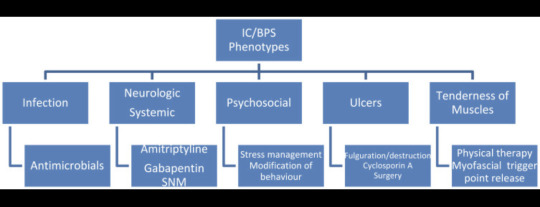
Conservative treatment, including patient education, behavioural modification, dietary advice, stress relief and physical therapy is an essential initial management strategy for all patients. If no response is observed, oral treatments such as amitriptyline are likely to offer the greatest response.
Cystoscopy is essential to phenotype patients, and Hunner lesion directed therapy with fulguration or resection can be performed at the same time. Intravesical instillation of DMSO or lidocaine, detrusor injections of botulinum toxin A and neuromodulation can be used if initial management fails to improve symptoms.
Oral cyclosporin can be trialled in those experienced with its use; however, it is associated with significant adverse events and requires intense monitoring. Lastly, radical surgery should be reserved for those with severe, unremitting BPS, in which quality of life is severely affected and not improved by previously mentioned interventions. Future work investigating exact aetiological factors will help target the development of efficacious treatment options, and several promising oral and intravesical treatments are emerging.
2 notes
·
View notes
Text
Upcoming New Drug Therapies for Nocturia - A Nonsystematic Stepwise Review- Juniper Publishers
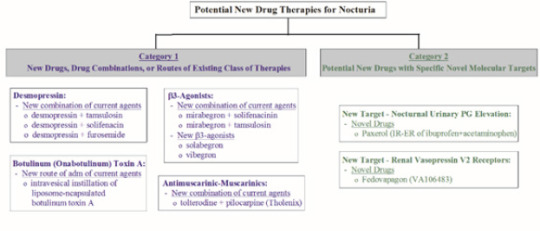
Juniper Publishers- Open Access Journal of Annals of Reviews & Research
Upcoming New Drug Therapies for Nocturia - A Nonsystematic Stepwise Review- Juniper Publishers
Authored by King C Lee
Abstract
Current treatment options for nocturia are unsatisfactory, prompting review of clinical studies of potential new and better drug therapies for nocturia. A 3-step nonsystematic review was performed. Step 1 was to review articles related to nocturia in multiple databases. Step 2 was to review articles identified in Step 1 for potential new and better drug therapies for nocturia. Step 3 was to review the websites of companies sponsoring new drug therapies. Two categories of potential new drugs were identified. Category 1 drugs include new drugs, new drug combinations, or new routes of administration of drugs that are in the existing class for nocturia. They are: (a) demospressin combined with tamsulosin (an alpha-1 blocker), solifenacin (an antimuscarinic), or furosemide (a diuretic); (b) mirabegron (a β3- agonist) combined with tamsulosin or solifenacin, or new β3-agonists (solabegron and vibegron); (c) tolterodine (an antimuscarinic agent) combined with pilocarpine (a short-acting muscarinic agonist selective for salivary gland receptors); and (d) intravesical instillation of botulinum toxin A. Category 2 drugs are new drugs with novel molecular targets. They include Paxerol (prostaglandin E2 inhibitors) and Fedovapagon (a vasopressin V2 receptor agonist).
Conclusion: Category 1 potential new drug therapies have improved efficacy and/or tolerability compared to parent drugs. Due to novel molecular targets, Category 2 drugs provide additional treatment options, especially in patients who have failed current therapies, found current therapies unsatisfactory, or cannot tolerate current drug therapies.
Keywords: Nocturia; Benign prostatic hyperplasia; Overactive bladder; Lower urinary tract symptoms; drug therapy; clinical trials
Abbreviations: QOL: Quality of Life; FDA: Food and Drug Administration; BPH: Benign Prostatic Hyperplasia; OAB: Overactive Bladder; LUTA: Lower Urinary Tract Symptoms; RCT: A randomized control trial; IPSS: International Prostate Symptom Score; PVR: Post Void Residual; EMA: European Medicines Agency; QD: Once daily; BID: Twice daily; ICSI: Interstitial Cystitis Symptom Index; ICPI: Interstitial Cystitis Problem Index; ADH: anti-diuretic hormone; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; NIH: National Institute for Health; PGE2: Prostaglandin
Introduction
Nocturia
Nocturia, together with etiology, pathology, risk factors, treatment and management, are thoroughly described by Lee & Weiss [1]. Briefly, nocturia is the complaint of waking at night to void, and clinically significant nocturia is 2-3 or more nocturnal voids [1,2]. This disorder is associated with significant mortality, morbidity, negative economic implications, reduced Quality of Life (QOL) [1-7]. Etiology is multifactorial with 3 commonly known mechanistic causes: global polyuria, nocturnal polyuria, and decreased nocturnal bladder capacity [1,8].
Current Therapy of Nocturia
The current therapies of nocturia include lifestyle and behavioural modifications as first line therapy.1 These initial measures are cumbersome and not sufficiently effective for most patients. Pharmacological therapies are introduced after first line therapy has failed or as adjuncts in patients with sustained bother from nocturia [9]. Among all drugs currently used to treat nocturia (Table 1), desmopressin is the only drug approved by Food and Drug Administration (FDA) for nocturia. The efficacy of desmopressin is marginal (0.2-0.4 nocturnal void reduction beyond those from placebo) and it is associated with a potentially serious adverse effect of hyponatremia. Other drugs for nocturia are off-label use, originally approved for treatment of Benign Prostatic Hyperplasia (BPH), Overactive Bladder (OAB), and/or other Lower Urinary Tract Symptoms (LUTS) [1]. They have limited clinical studies to support their use to treat nocturia and the clinical significance of some of these drugs is questionable [1,10]. Nocturia has a poor clinical response to traditional therapies for BPH and OAB [10]. The current drug therapies for nocturia are undoubtedly unsatisfactory. This article provides an overview of clinical studies of potential promising new drug therapies for nocturia.
Materials and Methods
A 3-step nonsystematic extensive review of articles was performed. Step 1 involved review of articles that are indexed in English in the PubMed, Google Scholar, and Embase databases. Key search terms were nocturia, nocturnal voids, OAB, BPH, incontinent, clinical studies, etc. Step 2 involved review of articles on potential new drug therapies identified from Step 1. The key search terms were the generic, code and trade names of potential new drugs. Step 3 involved review of the websites of companies that sponsor the new drug therapies.
Results
Overview of promising new drug therapies The potential new drug therapies for nocturia are in two categories (Figure 1). The first category includes new drugs, new drug combinations, or new administration routes of drugs that are in the existing class of drug therapies. These new drug therapies will undoubtedly be used to treat nocturia off-label, as in the case with approved drugs for OAB, BPH and LUTS. The second category includes potential new drugs that have novel molecular targets for nocturia.
Category 1 drugs
i. Desmopressin: Desmopressin, a synthetic form of vasopressin which reduces urine production within the kidney and is provided in nasal spray (Noctiva) and sublingual tablet (Nocdurna), was approved by FDA as monotherapy for nocturnal polyuria type of nocturia [11]. Given that desmopressin has limited efficacy (0.2-0.4 nightly void reduction beyond those from placebo) and nocturia is a multifactorial medical condition in most cases [1], a drug combination treatment can provide improved efficacy and/or broaden the application for the types of nocturia [12,13]. Several combinations have been investigated, as described in subsequent sections.
ii. Desmopressin and tamsulosin combination: A randomized control trial (RCT) was conducted to evaluate the combination of desmopressin and tamsulosin (an alpha-1 blocker) to treat nocturia in patients with BPH [14]. In this clinical study, 248 patients with nocturia associated with BPH were randomized into two groups. Patients received the sublingual formulation of desmopressin (60mcg/day) and tamsulosin (0.4mg/day), or tamsulosin (0.4mg/day) only, for 3 months. The results showed that, when compared to tamsulosin alone, the combination improved nocturia and prolonged the first sleep period. International Prostate Symptom Score (IPSS), QOL, Post Void Residual (PVR) urine volume, and maximum urinary flow rate at night were significantly improved compared to baseline, but not between the two groups. No serious adverse effects were reported in either group.
A RCT involving the use of desmopressin as an add-on therapy to tamsulosin was conducted to evaluate the desmopressin/ tamsulosin combination in the treatment of nocturia [15]. This clinical study enrolled 216 patients with nocturia, of whom 158 (76%) had nocturnal polyuria type of nocturia, 15 (7.2%) had decreased nocturnal bladder capacity type, and 35 (16.8%) had both types, despite tamsulosin treatment for ≥4 weeks. Individually optimized dose of oral desmopressin was added while patients were still on tamsulosin, and desmopressin+tamsulosin combination was maintained for the subsequent 24 weeks. The results showed that nocturnal voids were decreased, IPSS scores were improved, and the therapy was well tolerated.
iii. Desmopressin and solifenacin combination: A RCT investigating the effect of desmopressin combined with solifenacin (an antimuscarinic) has been conducted in 68 females with OAB [16]. Patients were treated with solifenacin (5mg) alone, or solifenacin (5mg) plus desmopressin (0.2mg), for 2 weeks. The results showed that there was no difference in the time to first void between the two groups, but time to the second and third voids were improved by the combination therapy compared to solifenacin alone. The combination therapy also improved the first urgency episode and QOL scores when compared to solifenacin alone.
iv. Desmopressin and furosemide combination: A RCT was conducted in 58 elderly men and 24 elderly women with nocturia ≥2 voids/night who received furosemide at 20 mg given 6 hours before bedtime plus individually optimized dose of desmopressin, or placebo plus desmopressin, for 3 weeks [17]. The results showed that the furosemide+desmopressin combination reduced nocturnal voids and nocturnal urine volume compared to furosemide+placebo.
β3-Agonists
i. Combination with mirabegron: Mirabegron, a β3- adrenoreceptor agonist, is the only FDA and European Medicines Agency (EMA) approved product in this class and is approved for treatment of OAB. Mirabegron is also used to treat nocturia associated with OAB off-label. Two combination therapies involving mirabegron have been conducted. The first combination, mirabegron+solifenacin (antimuscarinic agent), was investigated in a RCT (the BESIDE study) involving 2,110 OAB patients who remained to be incontinent (≥1 episode during 3-day diary) following 4-week single-blind once daily (QD) of solifenacin at 5mg [18]. The patients were then randomized into 3 groups of 1:1:1 ratio and treated for 12 weeks with: (a) 5mg solifenacin, (b) 10mg solifenacin, or (c) 5mg solifenacin with 25mg mirabegron during Weeks 1-3 and then 50mg mirabegron during Weeks 4-12. The results showed that OAB symptoms (based on daily micturition, urgency, and urgency incontinence) were reduced by mirabegron, solifenacin, and mirabegron+solifenacin combination, with the greatest improvement induced by the combination. Baseline nocturia was similar among the 3 groups (1.45±0.96 voids for 5 mg solifenacin, 1.50±1.03 for 10mg solifenacin, and 1.51±1.06 for mirabegron+solifenacin). Nocturia was eliminated in 100% subjects treated with 10mg solifenacin or combination, and 99.4% subjects treated with 5mg solifenacin. There were no notable differences in adverse events (AEs) among different treatments, with the exception of dry mouth being highest with 10mg solifenacin. These results showed that mirabegron+solifenacin combination was associated with the greatest improvement in OAB symptoms. For nocturia, mirabegron+solifenacin combination and 10mg solifenacin eliminated nocturia in all subjects, whereas 5mg solifenacin in most subjects. All 3 treatments were equally safe, although 10mg solifenacin induced a higher incidence of dry mouth.
The second drug combination investigated was mirabegron+tamsulosin (an alpha-1 blocker) as an add-on therapy [19]. This study enrolled 94, with 76 completed the study, BPH patients with OAB symptoms who had been treated with tamsulosin alone for ≥8 weeks. These patients were treated with tamsulosin (0.2mg), or mirabegron (50mg) + tamsulosin (0.2mg) combination, for 8 weeks. The results showed that OAB symptoms, based on OAB symptom scores (OABSS), were improved greater in patients treated with mirabegron+tamsulosin combination than tamsulosin alone (-2.21 vs. -0.87, P=0.012). Improvements in urinary urgency, daytime frequency, IPSS, QOL index, and PVR urine volume were also greater with the combination than tamsulosin alone. Compared to baseline, nocturia (assessed based on Question 7 of IPSS) was improved by the combination (prevs. post-treatment of 2.73±1.13 vs. 2.26±1.00, P=0.001) but not tamsulosin alone (2.47±1.10 vs. 2.31±1.14, P=0.225). AEs were observed in 6 patients treated with the combination, but not in patients treated with tamsulosin. In summary, this study showed that tamsulosin+mirabegron combination was effective in patients with BPH who have OAB symptoms and nocturia after tamsulosin monotherapy [20].
New β3-agonists. Two promising new selective β3- adrenoreceptor agonists (solabegron and vibegron) are being developed for the treatment of OAB, [21,22] and will undoubtedly be used to treat nocturia off-label. These β3-agonists largely lack the side effect of dry mouth, blood pressure or heart rate, and were generally safe [22-24]. For solabegron, a Phase 2 RCT was conducted to investigate the effectiveness of 50mg Solabegron (n=88) and 125mg solabegron (n=85), when compared to placebo (n=85), given twice daily (BID) for 8 weeks in incontinent women with OAB [23]. The results showed that, when compared to placebo, 125mg (but not 50mg) Solabegron significantly and consistently improved incontinence (P=0.025), micturition, and urine volume voided. Solabegron at both doses were well tolerated, with a similar AE incidence compared to placebo. Unfortunately, nocturia was not assessed in this study. These results were confirmed by a subsequent Phase 2b RCT (the VEL- 2002 trial) in 435 women with OAB treated with Solabegron given QD for 12 weeks, according to the website and press releases by Velicept Therapeutics (the sponsor of Solabegron). For vibegron, a Phase 2a RCT in 1,395 OAB patients has been conducted to assess 3, 15, 60, or 100mg of vibegron alone, or in combination with 4mg tolterodine (an antimuscarinic agent) [25]. All doses of vibegron given alone, or in combination with tolterodine, improved OAB symptoms (micturition, urge incontinence episodes, total incontinence episodes, and urgency episodes) when compared to placebo and were well tolerated. The incidence of dry mouth was higher with combination than vibegron monotherapy. No nocturia parameters were assessed in this study.
Also, for vibegron, a 12-week Phase 3 RCT was conducted to evaluate vibegron vs. placebo in 1,232 Japanese OAB patients [24]. Patients received one of the following treatments for 12 weeks: (a) vibegron, 50 or 100mg, QD; (b) placebo; or (c) imidafenacin, an anticholinergic agent used as a positive control, 0.1mg BID. Vibegron at both doses improved OAB symptoms (micturition/ day, daily episodes of urgency, urgency incontinence, incontinence, and voided volume/micturition) and QOL, when compared to placebo. Nightly voids were reduced from baseline by -0.58 void/ night by 50 mg vibegron and -0.62 void/night by 100mg, which were greater when compared to -0.47 void/night by placebo (P=0.016 and 0.001, respectively). A subsequent post-hoc analysis on 669 patients who had ≥1 nocturnal void showed that 50 and 100mg vibegron reduced the frequency of nocturnal voiding by 0.74 and 0.78, respectively, from baselines at Week 12, which was greater than placebo (P<0.05 and 0.001, respectively) [26]. Also, the mean volume of nocturnal voids and the volume of the first nocturnal voiding were greater in vibegron groups than placebo.
The vibegron groups showed significant correlations of longer hours of undisturbed sleep with greater reduction in the frequency of nocturnal voiding and higher volume of the first nocturnal voiding. AE incidences were similar among both doses of vibegron and placebo, and all were less than imidafenacin. This study showed that vibegron was effective and safe for patients with OAB symptoms and nocturia. Two Phase 3 RCTs of vibegron are ongoing to assess vibegron (75mg) on OAB symptoms in men who are receiving pharmacological treatment for BPH and yet continue to experience OAB symptoms. One trial (the COURAGE trial) compares vibegron vs. placebo treatments, with international study sites of intended sample size of approx. 1,000 men (Trial# NCT03902080) [27]. The other trial is a singlegroup trial comparing pre- vs. post-treatment with vibegron, with multiple study sites in the US of intended sample size of 300 men (Trial# NCT04103450) [28]. Both trials assess OAB symptoms as well as nocturia. If the results from these studies are positive, they would confirm the safety and efficacy of vibegron for OAB and nocturia in men with BPH.
ii. Antimuscarinic-muscarinic combination: Antimuscurinic agents are used to treat nocturia off-label see Table 1. Tholenix is a combination of tolterodine (an antimuscarinic agent) and pilocarpine (short-acting muscarinic agonist selective for salivary gland receptors) intended to minimize dry mouth without interfering with the efficacy of the antimuscarinic. A Phase 2 RCT showed that Tholenix caused less dry mouth compared with tolterodine alone, while maintaining full efficacy in patients with OAB [29]. A Phase 3 RCT on Tholenix confirmed that Tholenix and tolterodine alone had similar efficacy, with the combination having significantly lower rate of dry mouth than tolterodine alone [30]. These studies did not assess nocturia.
iii. Intravesical instillations of botulinum (onabotulinum) Toxin A: Intradetrusor injections of botulinum toxin-A (BTX-A) have been approved for treating OAB based on several RCTs [31]. It is also used to treat nocturia off-label. Several clinical studies have been conducted to assess the efficacy of BTX-A when administered by intravesical instillation [32]. The advantage of instillation is to reduce side effects and eliminate cystoscopic needle injection to facilitate BTX-A accessing the submucosal nerve plexus. A RCT showed that single intravesical instillation of liposome-encapsulated BTX-A decreases OAB symptoms [33]. This study did not access the treatment effect on nocturia.
Another study was conducted to assess the feasibility and safety of a mixture of TC-3 gel (a novel reverse-thermal gelation hydrogel) and BTX-A for the treatment of interstitial cystitis bladder pain syndrome (IC/BPS) administered by instillation. This study was conducted in 15 severely symptomatic patients who had Interstitial Cystitis Symptom Index (ICSI) and Interstitial Cystitis Problem Index (ICPI) scores ranged 12-19 and 12-16, respectively, and median Visual Analog Scale (VAS, for pain assessment) being 7. After instilled into the bladder as liquid, TC-3 gel/BTX-A mixture solidified because of body heat, and gradually dissolved to release BTX-A into the bladder over several hours via sustained release. The results showed that there was no increase in VAS score between pre- vs. post-treatment values (6.6±2.7 vs 5.3±2.8, P=0.044). All AEs were transient and mild, with the most common being temporary mild constipation (n=4, 26%). Mean ICSI and ICPI scores were reduced compared with baseline (15.4±2.4 vs. 12.9±4.3, and 14.8±1.4 vs. 11.9±4.0, both P=0.004). Compared to baselines, nocturia was decreased at Week 6 (3.3±2.1 vs. 1.8±0.9, P=0.046) and returned to baseline level at Week 12. The results indicated that intravesical instillation of a TC-3 gel/BTX-A mixture is effective, safe and tolerable for IC/BPS, and provided temporary relief of nocturia lasting for a few weeks.
Category 2 - drugs with novel molecular targets for treatment of nocturia
i. Urinary prostaglandin: Prostaglandin E2 (PGE2) is involved in the pathogenesis of nocturia, via inhibition of the hypothalamic anti-diuretic hormone (ADH) in the kidney which causes increases in urinary output and local modulation of reflex micturition in the bladder. ADH and PGE2 normally regulate the production of each other, thus keeping a fine balance of fluid hemostasis [34-36]. Urinary prostaglandin levels follow a circadian rhythm [37,38], and elevated urinary PGE2 levels occurred in patients with voiding diseases. Abnormal PGE2 levels at night occurred in patients with OAB [39,40]. Because of prostaglandins’ involvement in local modulation of reflex micturition and pathogenesis of nocturia, anti-prostaglandin agents should provide benefits in the treatment of nocturia. Indeed, various clinical studies have shown that Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), which are prostaglandin inhibitors, can improve nocturia symptoms (Table 2). All clinical studies were conducted using anti-inflammatory doses, which are several factors higher than those needed for urinary PGE2 inhibition. The presumption for the need of high or anti-inflammatory dose levels for antinocturia activities is understandable, as the role of inflammation in the pathophysiology of diseases associated with nocturia has been described [41,42].
Chronic use of NSAIDs at high or anti-inflammatory doses is associated with toxicity risk, including increase risk of cardiovascular and gastrointestinal toxicities, and risk of hepatotoxicity secondary to inadvertent overdose with acetaminophen (a prostaglandin inhibitor similar to NSAIDs). The National Institute for Health (NIH) and Clinical Excellence guidelines do not recommend the use of NSAIDs for treatment of nocturia. Paxerol is a novel proprietary immediate release/ sustained release (50%:50%) formulation of the prostaglandin inhibitors, acetaminophen and ibuprofen [43]. One-half of a Paxerol tablet is released via immediate release during the first hour to provide “loading dose” effect, whereas the other half is released via sustained release for up to 6 hours to coincide with the normal 6-8 hours of sleep. Paxerol is a low dose package form, with each tablet containing 325mg acetaminophen and 150mg ibuprofen. This is ~50% and ~12.5%, respectively, of the lowest daily anti-inflammatory dosages, and ~33% and ~5%, respectively, of maximum Over the Counter (OTC) daily doses. The amounts of ibuprofen and acetaminophen in each Paxerol tablet correspond to the lowest concentrations for maximum inhibitory effects of bladder smooth muscle contractions induced by lipopolysaccharide of Salmonella typhimurium in vitro (unpublished data). Furthermore, acetaminophen is a selective COX-2 inhibitor.
It is synergistic with ibuprofen in the inhibition prostaglandin production at the peroxidase (POX) site within COX-1/2 enzymes [44,45] and synergistic for the analgesic effects in humans [46,47]. Due to synergism, the use of low doses of ibuprofen and acetaminophen can maximize the efficacy for nocturia yet reduces the toxicity risks associated with long-term use. This is indeed the case, as demonstrated in a Phase 2 RCT of Paxerol in 86 patients with severe nocturia (≥2.5 nightly voids) associated with OAB [43] receiving one, two, or three Paxerol tablets per day for 14 days, or placebo. The 3 doses of Paxerol equally reduced nocturia when compared placebo. Percent of patients with an average baseline nocturnal void frequency of 3.6±1.0 episodes reduced to 0 or 1 void for ≤1 night were greater with the Paxerol groups than the placebo group. Also, ~35% of patients in this trial had BPH, as OAB and BPH are not exclusive etiologies. Subgroup analysis indicated that Paxerol appears to be equi-effective for those with and without BPH. There were no treatment related AEs in any groups. Note that although safety and broad efficacy were demonstrated, this Phase 2 RCT was a short-term study with limited sample size of 86. Further dose-response studies with larger sample size and for longer treatment duration are needed to validate the efficacy and safety of Paxerol to treat nocturia.
ii. Renal vasopressin V2 receptor: Fedovapagon, a vasopressin V2 receptor agonist, is a novel small molecule investigational drug for the treatment of nocturia. Fedovapagon induces antidiuretic effect by activating the vasopressin V2 receptors in the collecting ducts of the kidney. Activation of the V2 receptors causes the kidneys to reabsorb water from urine as it passes toward the bladder. Dosing of fedovapagon before bedtime leads to less urine produced overnight. Clinical studies showed that fedovapagon induced dose-dependent reductions in nighttime urine production and urine volumes. Fedovapagon has a longer half-life than desmopressin, which may make the drug more likely to result in prolonged antidiuresis at nighttime compared with desmopressin but could result in a higher risk of hyponatremia.
A Phase 2/3 RCT (the EQUINOC trial) in 432 patients with BPH and nocturia has been conducted [48,49]. Patients received PO fedovapagon (2 mg) or placebo each evening over 12 weeks. When compared to placebo, fedovapagon improved nocturnal voids from baseline (p=0.004). There were also improvements in time to first void (p<0.001), nights when patients had 0 or 1 voids (p=0.038), and patients who reduced nightly voids by 50% (P=0.007). Fedovapagon was generally well tolerated. This study indicated that Fedovapagon may be an effective treatment for BPH and nocturia. A second Phase 3 study based on the same endpoints and patient population is ongoing. The goal is to provide additional data required to support regulatory filings for marketing approval.
Discussion and Conclusion
Clinical studies of two categories of potential new drug therapies for nocturia are described in this review article. Category 1 drugs are new drugs, new drug combinations, or new administration routes of drugs that are in the existing class of drug therapies for nocturia see Table 1. They improved efficacy, safety or tolerability. β3-Adrenoreceptor agonists were one type of Category 1 drug therapies. Mirabegron is currently the only FDA and EMA approved drug in this class, with the approved indication for treatment of OAB. Their use to treat nocturia associated with OAB is off label. There are recent clinical studies of three new β3- adrenoreceptor agonists (solabegron, vibegron and ritobegron). The β3-agonists largely lack the side effects of dry mouth, had no risk for blood pressure or heart rate increase, and were generally safe [22-24]. This review article describes only solabegron and vibegron. For ritobegron (KUC-7483), only Phase 1 trial data were published [50]. The Phase 2 and Phase 3 RCTs (Study Nos. NCT00742833 and NCT01003405, respectively) [51,52] for assessments of OAB are listed as “COMPLETED” in clinicaltrials. gov. Another Phase 3 RCT (Study No. NCT01003415) [53], also for assessment of OAB, is listed as “WITHDRAWN”. Data from these Phase 2 and 3 RCTs were not published. On the website of the sponsor (Kissei Pharmaceutical Co., Ltd), the first Phase 3 trial was once labeled as having failed to reach its primary endpoint and the program has been put on hold [54].
The sponsor might have terminated clinical development of this product. Also, emerging pharmacovigilance data on mirabegron have identified a rare risk for excessive hypertension, thus are contraindicated in patients with severe hypertension [55]. The excessive hypertensive effect of mirabegron is not related to activation of cardiac β3-adrenoceptors, as the human heart lacks functional β3-adrenoceptors [56], but may be due to β1-adrenoceptor medicated positive inotropic effects on human atrium [57]. It is unknown if the new selective β3-agonists also induce the rare severe hypertension. Another type of Category 1 drug therapy is the antimuscarinic/muscarinic combination. Tholenix is a combination of tolterodine (an antimuscarinic agent) and pilocarpine (a short-acting muscarinic agonist with selectivity for salivary gland receptors) for countering the dry mouth without interfering with the efficacy of the antimuscarinic. Phase 2 and 3 RCTs showed that Tholenix caused less dry mouth compared with tolterodine alone, while maintaining full efficacy on OAB symptoms [29,30]. These studies did not assess nocturia. However, clinical studies have shown that tolterodine alone, though effective for OAB, is not effective for nocturia [1,58]. Therefore, tolterodine+pilocarpine combination is unlikely to be effective for nocturia.
Category 2 drugs are those with novel molecular targets. These drug therapies can provide additional treatment options, especially in patients who have failed current therapies, found current therapies unsatisfactory, or cannot tolerate current drug therapies. Two investigational drugs (Paxerol and fedovapagon) were described in the current article, both with promising clinical trial results. Paxerol has a novel proprietary immediate release/ sustained release (50%:50%) formulation of the prostaglandin inhibitors, acetaminophen and ibuprofen [43]. As expected, Paxerol is safety and well tolerated, due to the doses of the prostaglandin inhibitors being a fraction of OTC doses used for anti-inflammatory and analgesic indications. Paxerol’s nocturia efficacy is broad, encompassing males and females with OAB and/ or PBH. This is also expected as its site of action is “downstream”, located in the bladder. However, Paxerol’s clinical data are from a short-term Phase 2 RCTs with limited sample size of 86. Further RCTs are needed. The second potential drug therapy with a novel molecular target is fedovapagon, a potent vasopressin V2 receptor agonist. The second Phase 3 study in patients with nocturia is ongoing and is needed to support regulatory filings for marketing approval. Fedovapagon induces antidiuresis at nighttime which can lead to hyponatremia, as in the case with desmopressin. This potential AE is being carefully monitored in the ongoing Phase 3 trial [59-72].
Summary and Conclusion
There are a number of potential new drug therapies for nocturia. One category includes the new drugs, new drug combinations, or new administration routes of drugs that are in the existing class of drug therapies for nocturia. Category 1 drug therapies frequently resulted in improvement of efficacy and/or tolerability. The second category of potential new therapies has novel molecular targets. Category 2 drugs can provide additional treatment options, especially in patients who have failed current therapies, found current therapies unsatisfactory, or cannot tolerate current drug therapies. The Category 2 drugs include Paxerol (a combination of PGE2 inhibitors) and fedovapagon (a vasopressin V2 receptor agonist).
To know more about Juniper Publishers please click on: https://juniperpublishers.com/aboutus.php
For more articles in Open Access Journal of Reviews & Research please click on: https://juniperpublishers.com/arr/index.php
To know more about Open Access Journals please click on: https://juniperpublishers.com/journals.php
#Juniper Publishers#Juniper Publisher Reviews#Juniper Publishers group#juniper publisher journals#Juniper Publishers Indexing List
0 notes
Text
Aztreonam


Common Brand Names: Azactam
Therapeutic Class: A monobactam antibiotic
Common Injectable Dosage Forms:
Powder for Injection (Lyophilized cake): 500 mg, 1 g, 2 g as either vials for injection or 100 mL infusion bottles
Frozen Premixed Solution: 1 g, 2 g
Dosage Ranges:
For the treatment of lower respiratory tract infections (including pneumonia and bronchitis), septicemia, skin and skin structure infections (including those associated with postoperative wounds or ulcers or burns), intra-abdominal infections (including peritonitis), and gynecologic infections (including endometritis and pelvic cellulitis) caused by susceptible gram-negative bacteria: 1-2 g every 8-12 hours.
For the treatment of complicated and uncomplicated urinary tract infections (including pyelonephritis and cystitis): 500 mg or 1 g every 8-12 hours.
For the treatment of moderately severe systemic infections: Adults should receive 1-2 g every 8-12 hours. Severe systemic or life-threatening infections, especially those involving P. aeruginosa, may require 2 g every 6-8 hours. The maximum adult dosage recommended is 8 g/day.
Pediatric dosage (1 month to 15 years) has been established at 30-59 mg/kg every 4-8 hours.
Administration and Stability:
For direct IV injection, the dose should be diluted with 6-10 mL of diluent and injected over a period of 3-5 minutes. For intermittent IV fusion, aztreonam should be diluted with a compatible solution to a final concentration not exceeding 20 mg/mL and infused over a period of 20-60 minutes. For IM injection at least 3 mL of an appropriate diluent should be added per gram of aztreonam and injected deeply into a large muscle. pH: 4.5-7.5
Pharmacology/Pharmacokinetics:
Aztreonam is a synthetic monobactam antibiotic that exhibits its bactericidal effect by inhibiting bacterial cell wall synthesis. Aztreonam is active against many gram-negative bacteria, including most Enterobacteriaceae and P. aeruginosa, but has little or no activity against gram-positive or anaerobic bacteria. Aztreonam is 100% bioavailable when given IM, producing peak serum levels in 1 hours. The drug is excreted primarily unchanged in the urine and has an elimination half-life of 1.7 hours, which is prolonged in renal failure, requiring dosage adjustments.
Drug and Lab Interactions:
Aztreonam, like most cephalosporins, can produce false-positive Benedict’s test for urine glucose. The antibacterial activity is additive or synergistic with aztreonam is combined with other antibiotics such as aminoglycosides, beta-lactams, and clindamycin, and should always be used in combination in the treatment of mixed infections, or those of unknown etiology, to ensure coverage gram-positive and anaerobic bacteria.
Contraindications/Precautions:
Care should be taken to determine if patients have any previous history of hypersensitivity to any beta-lactams, as some degree of cross-sensitivity has been demonstrated. Aztreonam appears to lack the nephrotoxic effects seen with aminoglycosides, but elevations in hepatic function tests have been frequent. As with other antibiotics, superinfections are a concern during aztreonam therapy. Pregnancy Category B.
Monitoring Parameters:
LFTs, BUN, Cr
Adverse Effects:
Nausea, vomiting, diarrhea, and other GI effects have been reported with aztreonam therapy. Skin rash, phlebitis, and blood dyscrasias have also been reported as other adverse effects associated with the use of aztreonam.
Common Clinical Applications:
An effective antibiotic against gram-negative bacteria. Usually used in combination with another antibiotic in order to cover gram-positive infections.
0 notes
Text
Interstitial Cystitis A Painful Bladder Syndrome
Interstitial cystitis is one of the major clinical syndromes characterized by daytime and nighttime urinary frequency, urgency, and pelvic pain of unknown etiology.
Besides, interstitial cystitis (IC) is commonly found in middle-aged women, whose characteristics are mainly the fibrosis of the bladder wall. Through the above simple thought, we should be aware that if one is looking to treat the interstitial cystitis, she must first understand the circumstances of interstitial cystitis. Generally recognized as an antidote against the disease.
Causes Of Cystitis
All patients who had normal urine and infection was not the main cause of bladder wall fibrosis. Some scholars believe that the lymphatic obstruction caused by pelvic surgery or infection is the cause of the disease, and many patients do not have such a history.
Some scholars have suggested that it may be related to endocrine factors because of the acute infection of the patients with the disease of the urinary bladder or pelvic organs, or due to the spasm of the long term of the mental impulse.
There are some causes of interstitial cystitis, as follows: First, the infection. The urine culture of interstitial cystitis patients contains a few bacteria, antibiotic therapy is often ineffective. Second, inflammation is the main manifestation of interstitial cystitis. Third, mast cells and activation. Also, toxic substances and hypoxia can cause damage to the bladder, then lead to interstitial cystitis. Interstitial cystitis has no clear etiology or pathophysiology, and diagnostic and other criteria for the syndrome remain undefined and usually unclear.
Despite considerable research in the respective field, universally effective treatments do not exist; therapy usually consists of various supportive, behavioral and pharmacologic measures. Some people think that the Diuretic and anti-inflammatory pill is a good treatment choice.
The Diagnosis Of Interstitial Cystitis
The diagnosis of interstitial cystitis mainly depends on symptoms, physical examination, urine analysis, bladder dilation, and biopsy. Under general anesthesia, taking a cystoscopy can make diagnosis of interstitial cystitis. The appearance and volume of the bladder were normal, but when refilling the bladder after emptying it, then often could be seen scattered in mucosa bleeding biopsy showed edema, hyperemia, telangiectasia and blood vessels around the hemorrhage of pathological change, which also can be used to exclude some carcinoma in situ and tuberculosis lesions in the lower epidermis.
Talking About Treatment
At present, the treatment effect of interstitial cystitis is unsatisfactory, 90% of the cases of conservative treatment can alleviate the symptoms, 10% of them need for surgical treatment. The usual treatments are: Behavioral therapy, which means the control of fluid intake and pelvic floor muscle training. There is drug treatment, such as sedative, glucocorticoid and anti-allergy drugs, but the treatment effect is not ideal and the side effect is big, so doctors do not recommend to use. However, the Diuretic and anti-inflammatory pill can play a large role in treating the interstitial cystitis, and it has little side effect. At the same time, the pill has a good resistance to drugs.
Resource Box
Meet one of the best urologists in the Delhi-NCR and you can get unmatchable advice.
Content source : https://draksainiurologistindelhi.home.blog/2019/10/15/interstitial-cystitis-a-painful-bladder-syndrome/
0 notes
Link
Female Sexual Dysfunction & Diabetes
Causes of FSD in DiabetesVascular damage can affect blood slupply to the vagina and clitoris which can cause problems with dryness and arousal.Neuropathy can reduce sensitivityDiabetes can also lead to low oestrogen levels which can also affect the lubricationDepression can lead to FSD in a diabetic women and can affect relationships.
FSD in the Diabetics Assessed in a Survey
Female sexual response cycle is a complex non linear progression from desire to arousal and orgasm.Diabetes particularly affects arousal with decreased genital sensation and lubrication.Vaginal dryness & infections may lead to dyspareunia.Sexual functioning can be affected by Vaginitis caused by yeast infection and cystitis often result of a UTI.Predictors of sexual dysfunction in women include depression.
Neither age, duration of diabetes, glycemic control nor complications in predict sexual dysfunction in women as they do in men.Low androgens and possibly estrogens may be etiologic, as may numerous medications used by patients with diabetes.Recognition of the high prevalence of FSD (up to
Results show that women with type one diabetese, depression and marital status are the main predictors of FSD, whereas glycaemic control & complications were not associated with FSD.Further studies are needed to elucidate the mechanisms underlying these differences.Considering that FSD can have an important negative effect on quality of life and partner relationships, the sexual difficulties of women with diabetes warrant more attention in both research & practice.50%) & potential increase, in tandemwith that of diabetes, is needed.
In. The absence of definitive treatment, psychosexual counselling, relationship & sex therapy, DHEA supplements, vaginal lubricants, Flibanserin, low doses of estrogens or androgens, and Vitamin T(touch) have been used toRelieve the personal distress of FSD.
Conclusion- FSD is more frequent in diabetic than in control women, but it is still poorly understood; low Female Sexual Function Index is associated with high BMI.Sexual functioning of women with diabetes, has received far less attention in research, and results are less conclusive than those of studies in men. Further studies are necessary to better understand the risk factors for FSD in diabetic women.
Read More...Best Sexologist in Jaipur, Sexology Hospital in Jaipur | Vivan Hospital
0 notes
Text
Adenovirus Diagnostic Testing Market 2026 Consumption Analysis by Recent Trends, Development Forecast, and Applications

Adenovirus is one of the DNA viruses that are considered to be a major cause of febrile illness, primarily among children. Individuals and Infants with weak immune systems, cardiac disease or chronic respiratory problems are at higher risk of developing adenovirus infection, however, most infections are not severe. Adenovirus is a communicable infection, as it is an air-borne diseases and can spread from infected person to others by coughing and sneezing, and close contact such as shaking or touching hands. Adenovirus can cause pneumonia, acute respiratory disease, epidemic kerato-conjunctivitis, acute follicular conjunctivitis, gastroenteritis, and cystitis. Among infants, pharyngeal-conjunctival and pharyngitis fever are commonly caused by Adenovirus. In 2016, a study conducted by Barcelona Center for International Health Research and University of Barcelona, on children suffering from pneumonia, suggests that adenovirus is the second-most common respiratory virus causing pneumonia. Some adenoviruses can also spread through the infected person’s stools, for instance, during diaper changing. Adenovirus can also spread through water such as swimming pools, however, this is less common. Adenovirus infections can be usually diagnose by molecular methods or can be detected with polymerase chain reaction (PCR), serology, antigen detection or viral culture. Others emerging technologies such as liposomes, monoclonal antibodies, chromatography, flow cytometry, and gel micro droplets are also of vital use in diagnosis of adenovirus infection.
Request Sample Copy of Research Report @ https://www.coherentmarketinsights.com/insight/request-sample/1678
Adenovirus Diagnostic Testing Market – Driver
Adenoviruses are associated with a variety of nonspecific manifestations and clinical syndromes, thus diagnosis based upon clinical criteria alone is challenging. Diagnosis is most accurate when the infection shows outbreak symptoms or individuals show serious disease manifestations. Validation of adenovirus infection is essential for identifying the most appropriate treatment to establish a prognosis and initiate infection control measures by using correct antiviral agents, thus making diagnosis of these viruses a vital procedure. This in turn, is expected to augment growth of the adenovirus diagnostic testing market. Generally, there are two types of adenoviruses namely, Type 4 and Type 7 that caused severe outbreaks of respiratory illness, especially among military recruits. Thus, adenovirus vaccine, containing Type 4 and Type 7, is thus approved for military personnel aged 17 to 50 years from 1971, to protect them against the illness caused by these two viruses. In the recent past, adenovirus infections were diagnose by cell culture, as the virus replicates efficiently in cell cultures. Monoclonal antibodies are also used to detect infected cells using direct fluorescence antibody assays. Amplification and detection of adenovirus DNA by polymerase chain reaction methods are also gaining popularity as laboratory methods to determine adenovirus infection. The rapid detection and quantitation of adenovirus DNA, especially by a sensitive PCR technique is expected to aid in the diagnosis and treatment monitoring of adenovirus infections, particularly in immunocompromised patients. This in turn, is expected to boost growth of the adenovirus diagnostic testing market during the forecast period.
Moreover, growing research and development of technologies for use in the diagnosis of acute respiratory infections of viral etiology is expected to fuel market growth. For instance, in 2014, DiaSorin launched its sixth LIAISON test for the qualitative detection of adenovirus in stool samples in markets outside the U.S. and the U.K., the test is an addition to the 5 most important tests of the stool testing panel, already available in the market (C. Difficile toxin A&B, C. Difficile GDH, Helicobacter Pylori, EHEC, and Rotavirus).
Adenovirus Diagnostic Testing Market – Regional Analysis
Geographically, the global adenovirus diagnostic testing market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. The market in North America is expected to gain significant traction, owing to growing public health concerns regarding various infectious diseases such as pneumonia, which are associated with adenovirus infection. For instance, in 2014, the eighth cause of mortality in the U.S. was influenza and pneumonia together, as reported by the National Centre for Health Statistics. The adenovirus diagnostic testing market is expected to gain traction in Africa markets, where a large number of children infected with adenovirus are underserved.
Inquire Here Before Purchase of Research Report @ https://www.coherentmarketinsights.com/insight/talk-to-analyst/1678
Adenovirus Diagnostic Testing Market – Competitive Landscape
Manufactures are offering various immunoassays for the detection of adenovirus antigen. SD BIOLINE Rota/Adeno Ag test, is one of the immune-chromatographic assays offered by Abbott, for the qualitative detection of the presence of rotavirus or adenovirus antigen in human fecal specimens. Furthermore, in 2017, Abbott collaborated with University of California, San Francisco (UCSF) to discover and characterize novel viruses as well as develop diagnostic tools to address the potential health threats cause by adenoviruses. Vendors also focusing on improving the quality of their assays by conducted surveillance projects at various hospitals, which in turn, is expected to create lucrative opportunities for growth in this market. For instance, BioMérieux SA conducted surveillance of rotavirus in hospitals and health centers in Niger from 2010 to 2012, using the RDT VIKIA Rota-Adeno assay to check their product accuracy. After receiving concerns regarding this test, company subsequently performed a parallel evaluation of the diagnostic accuracy of VIKIA Rota-Adeno RDT test assay, which is a good alternative for use in peripheral health centers where laboratory capacity is limited.
Player operating in the adenovirus diagnostic testing market include Affymetrix, Inc., Becton Dickinson and company, F. Hoffmann-La Roche AG, Bio-Rad Laboratories Inc., Novartis AG, Qiagen N.V., DiaSorin Inc., Abbott Laboratories, and bioMerieux.
0 notes
Text
Adenovirus Diagnostics Market Poised to Expand at a Robust Pace by 2026
Adenovirus is one of the DNA viruses that is considered to be the primary cause of fever illnesses, mostly among children. Individuals and children with chronic respiratory complications, cardiac disease, or weak immune systems are at higher risk of contracting adenovirus infection. However, most of the infections caused by adenovirus are not too severe. Adenovirus is a contagious infection, as it is an airborne disease and can spread from a person who is already infected to others through sneezing, cough, and close contact. Adenovirus can cause acute respiratory disease, pneumonia, epidemic keratoconjunctivitis, gastroenteritis, acute follicular conjunctivitis, and cystitis. Among children, pharyngitis fever and pharyngeal-conjunctival are the most common infection caused by adenovirus.
Read Report Overview: https://www.transparencymarketresearch.com/adenovirus-diagnostic-market.html
A research conducted by the Barcelona Center for International Health Research and the University of Barcelona in 2016 indicated that adenovirus is the second most common respiratory virus that causes pneumonia. Few strains of adenoviruses can also spread through the infected person’s stool. Adenovirus can also spread through water such as swimming pools; however, this is not common. Infection caused by adenovirus can be usually diagnosed by molecular methods or detected with serology, polymerase chain reaction (PCR), viral culture, or through antigen detection. New technologies such as liposomes, chromatography, monoclonal antibodies, gel microdroplets, and flow cytometry are emerging as the methods of choice for the diagnosis of adenovirus infection.
Authentication of the infection caused by adenovirus is important to identify the most suitable treatment in order to establish a prediction and start a treatment procedure to control the infection by the application of accurate antiviral agents. This, in turn, is expected to boost the growth of the global adenovirus diagnostics market. In the recent past, adenovirus infections were diagnosed by cell culture, as the virus replicates efficiently in cell cultures. Detection and amplification of adenovirus DNA by different PCR methods are becoming the most widely accepted laboratory procedures for the detection of adenovirus infection. The swift detection and quantitation of adenovirus DNA, especially by techniques such as PCR, are expected to support the identification and treatment monitoring of adenovirus infections, particularly in immune-compromised patients. This, in turn, is expected to boost the growth of the global adenovirus diagnostic market during the forecast period. Moreover, increase in investment in research and development and technological advancements in the diagnosis of acute respiratory infections of viral etiology are expected to boost adenovirus diagnostics market growth.
Request Brochure of Report: https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=1456
The global adenovirus diagnostics market can be segmented based on diagnostic method and end-user. In terms of diagnostic method, the adenovirus diagnostics market can be categorized into viral culture, polymerase chain reaction assays and antigen-based assays, monoclonal antibodies, liposomes and flow cytometry, chromatography, and gel microdroplets. Based on end-user, the global adenovirus diagnostics market can be divided into hospitals, private diagnostic laboratories, small clinics, and public health laboratories.
In terms of region, the global adenovirus diagnostics market can be segmented into five major regions: North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. The adenovirus diagnostic market in North America is expected to gain significant traction, owing to increase in public health concerns regarding various infectious diseases such as pneumonia, which is associated with adenovirus infection and reimbursement for adenovirus diagnostics. Moreover, there are a number of hospitals and private diagnostics laboratories in the U.S. and Canada that offer a broad portfolio of tests to determine the presence of adenovirus. Europe held significant share of the global adenovirus diagnostics market. The adenovirus diagnostics market in Asia Pacific is expected to grow at a rapid pace due to rise in prevalence of the infection and large population. The adenovirus diagnostic market is expected to gain traction in Africa, where a large number of children infected with adenovirus are underserved.
Request For TOC : https://www.transparencymarketresearch.com/sample/sample.php?flag=T&rep_id=1456
Key players in the global adenovirus diagnostics market include Agilent Technologies, Beckman Coulter/Danaher, Becton, Dickinson and Company, Affymetrix, Bio-Rad, Roche, Novartis AG, Gen-Probe, Qiagen, Abbott, DiaSorin, and bioMerieux.
About us:
Transparency Market Research (TMR) is a U.S.-based provider of syndicated research, customized research, and consulting services. TMR’s global and regional market intelligence coverage includes industries such as pharmaceutical, chemicals and materials, technology and media, food and beverages, and consumer goods, among others. Each TMR research report provides clients with a 360-degree view of the market with statistical forecasts, competitive landscape, detailed segmentation, key trends, and strategic recommendations.
Contact us:
Transparency Market Research
90 State Street,
Suite 700,
Albany
NY – 12207
United States
Tel: +1-518-618-1030
USA – Canada Toll Free 866-552-3453
Email: [email protected]
Website: http://www.transparencymarketresearch.com/
0 notes
Text
Cystitis Etiology
-- E. coli
-- Klebsiella
-- Proteus
-- Enterococcus
-- Staph saprophyticus
-- Group B strep
-- Pseudomonas
-- Adenovirus
Patreon | Ko-fi
#studyblr#notes#medblr#medical notes#med notes#cystitis#cystitis notes#cystitis etiology#cystitis causes#etiology#causes#diseases and disorders#diseases#diseases notes#pathology#pathology notes#pathophysiology#pathophysiology notes#biology#science#science notes#biology notes
2 notes
·
View notes
Text
Dating with adrenal fatigue
How To Recover From Extreme Burnout (Adrenal Fatigue, Exhaustion)
Clinical outcome can be very positive and dramatic. This is when the amount of sodium falls relative to the increase in the amount of fluid and is a no-win situation for the sufferer. It is followed by the insulin-producing portion of the pancreas, then the thyroid, ovaries, parathyroid pineal, pituitary, and finally, the link to the autonomic nervous system, the hypothalamus. These include low blood pressure and frequent urination. Typical presentations in females involve symptoms of under-active thyroid, unbalanced ovarian hormones, and low adrenal function.
Adrenal fatigue: Signs and symptoms explained by Jess Blair
Water also regulates our body temperature and metabolism. Often, instead of taking advantage of these hints to help us gauge the level of dysfunction and use them as markers during the recovery process, the tendency is to turn to the quick fix approach of suppressing these symptoms. A body with a low internal cortisol level will succumb to frequent infections, have greater frequency of allergies and reduced autoimmune response. Eat a , and try some of these. Related Videos about Best Herbs for Energy and Preventing Fatigue : Feeling Tired All the Time? Examples include the inability to recall where keys are placed, what was done yesterday, difficulty in concentrating, difficulty in memorizing things such as telephone numbers, and confusion. This hyperactivity can fragment sleep patterns and decreases slow-wave sleep. It comes as no surprise that people in this phase of Adrenal Fatigue complain of an increase in unclear thinking, difficulty in concentrating, anxiety, food allergies, multiple chemical sensitivity, food intolerance especially dairy, wheat, and corn products, all of which are hard to digest , pain of unknown origin, insomnia, anxiety, food coma, increase in the incidence of yeast infection, candidiasis, irritable bowel, chronic fatigue and interstitial cystitis among others.
DATING ADVICE: How to overcome dating fatigue? (Dating advice for guys)
Over the following two weeks I subjected myself to a battery of tests from doctors, naturopaths, and other healers… and nothing showed up. Adrenal fatigue is fast of the 21st century. The productive hours of the day will progressively decrease and it is not unusual for those at this stage of Adrenal Fatigue to have only a few hours of productive time a day, while the rest of the time is spent in bed resting. Current concepts point to the likelihood of pump failure, receptor site dysfunction, transport system dysregulation, low clearance due to incomplete detoxification, metabolite buildup, Autonomic Nervous System reactive responses, and electron driven retoxification as possible etiology for the wide variety of paradoxical and exaggerated reactions commonly seen in this phase. A common remedy for busy moms with children and busy working moms with no time for themselves. Without sufficient levels of hormones, the body goes into a full-blown shut down mode to stop as much of the non-essential functions as possible to conserve energy in order to survive.
Juicing and Fasting for Adrenal Fatigue
Stage 3: Adrenal Exhaustion Adrenal Exhaustion refers to Stage 3 of Adrenal Fatigue. If your blood pressure drops, you likely have adrenal fatigue. Red blood cells carry oxygen throughout the body, which aids energy production in the cells. The body as a whole, however, is still functioning relatively well. This imbalance causes another set of metabolic problems further complicating the picture and the effects of Adrenal Fatigue. Some Adrenal Fatigue crashes will progress steadily over time, from Stage 1 to Stage 3 slowly, while others jump quickly into Stage 3 and never fully recover.
Adrenal fatigue: What causes it?
During college I worked hard, hardly slept and hardly ate. Reach out for help to friends and family. With each hypoglycemic episode, more cells are damaged. It may also interfere with some drugs. Do you find yourself constantly fatigued, and struggling to get out of bed in the mornings? Long-term memory, however, remains intact.
Juicing and Fasting for Adrenal Fatigue
Many suffering from Adrenal Exhaustion will report having low blood pressure as well as a salt craving. The low blood pressure is due to the reduced amount of fluid in the body and the salt craving is because the body is in an absolute deficiency of sodium. Yes, people will be disappointed with you. The condition will only get worse if the symptoms are not arrested. In other words, even though the brain is requesting more cortisol to be made, there is a blunted response from the adrenals to meet this request. The body initially produces large amounts of cortisol to cope with the stressors, but eventually cortisol levels drop.
How To Recover From Extreme Burnout (Adrenal Fatigue, Exhaustion)
Try adding ½ teaspoon of Rhodiola powder to your daily smoothie or coffee. Normal body function requires optimum and timely processing of nutrients by the liver once they are ingested and absorbed in the gut. Clinically, Adrenal Exhaustion can broadly be categorized into four overlapping phases that are decidedly indistinct. The adrenal glands however, have developed a blunted response over time to such continued stimulation strategy. Take licorice root as a standard adaptogen to help balance your cortisol levels. Decline in adrenal function is slow and steady and sometimes this is written off as the effect of aging. The bag I have lasts me for several months.
Juicing and Fasting for Adrenal Fatigue
Without further ado, here are the twelve highest leverage things that I did to recover from my bout of extreme burnout. Toxins cleared by the liver include alcohol, solvents, formaldehyde, pesticides, herbicides and food additives. Overall, body clearance of such by-products tends to slow concurrently as toxic metabolite build up occurs as a result. The low energy state renders the patient bed-ridden most of the time. This adaptogen is also anti-inflammatory, modulates the immune system, is antioxidant, anti-viral and helps expel excess phlegm. Depending on which stage of Adrenal Fatigue you have reached, you may be experiencing a handful or a large number of these symptoms. In healthy individuals, rises when rising from a laying position.
0 notes
Text
Adenovirus Diagnostics Market Analysis by Recent Trends, Development and Growth Forecast by Regions and Applications to 2026
Adenovirus is one of the DNA viruses that is considered to be the primary cause of fever illnesses, mostly among children. Individuals and children with chronic respiratory complications, cardiac disease, or weak immune systems are at higher risk of contracting adenovirus infection. However, most of the infections caused by adenovirus are not too severe. Adenovirus is a contagious infection, as it is an airborne disease and can spread from a person who is already infected to others through sneezing, cough, and close contact.
View Report: https://www.transparencymarketresearch.com/adenovirus-diagnostic-market.html
Adenovirus can cause acute respiratory disease, pneumonia, epidemic keratoconjunctivitis, gastroenteritis, acute follicular conjunctivitis, and cystitis. Among children, pharyngitis fever and pharyngeal-conjunctival are the most common infection caused by adenovirus. A research conducted by the Barcelona Center for International Health Research and the University of Barcelona in 2016 indicated that adenovirus is the second most common respiratory virus that causes pneumonia. Few strains of adenoviruses can also spread through the infected person’s stool. Adenovirus can also spread through water such as swimming pools; however, this is not common. Infection caused by adenovirus can be usually diagnosed by molecular methods or detected with serology, polymerase chain reaction (PCR), viral culture, or through antigen detection. New technologies such as liposomes, chromatography, monoclonal antibodies, gel microdroplets, and flow cytometry are emerging as the methods of choice for the diagnosis of adenovirus infection.
Authentication of the infection caused by adenovirus is important to identify the most suitable treatment in order to establish a prediction and start a treatment procedure to control the infection by the application of accurate antiviral agents. This, in turn, is expected to boost the growth of the global adenovirus diagnostics market. In the recent past, adenovirus infections were diagnosed by cell culture, as the virus replicates efficiently in cell cultures. Detection and amplification of adenovirus DNA by different PCR methods are becoming the most widely accepted laboratory procedures for the detection of adenovirus infection. The swift detection and quantitation of adenovirus DNA, especially by techniques such as PCR, are expected to support the identification and treatment monitoring of adenovirus infections, particularly in immune-compromised patients. This, in turn, is expected to boost the growth of the global adenovirus diagnostic market during the forecast period. Moreover, increase in investment in research and development and technological advancements in the diagnosis of acute respiratory infections of viral etiology are expected to boost adenovirus diagnostics market growth.
The global adenovirus diagnostics market can be segmented based on diagnostic method and end-user. In terms of diagnostic method, the adenovirus diagnostics market can be categorized into viral culture, polymerase chain reaction assays and antigen-based assays, monoclonal antibodies, liposomes and flow cytometry, chromatography, and gel microdroplets. Based on end-user, the global adenovirus diagnostics market can be divided into hospitals, private diagnostic laboratories, small clinics, and public health laboratories.
Request a Brochure of the Report @https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=1456
In terms of region, the global adenovirus diagnostics market can be segmented into five major regions: North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. The adenovirus diagnostic market in North America is expected to gain significant traction, owing to increase in public health concerns regarding various infectious diseases such as pneumonia, which is associated with adenovirus infection and reimbursement for adenovirus diagnostics. Moreover, there are a number of hospitals and private diagnostics laboratories in the U.S. and Canada that offer a broad portfolio of tests to determine the presence of adenovirus.
Europe held significant share of the global adenovirus diagnostics market. The adenovirus diagnostics market in Asia Pacific is expected to grow at a rapid pace due to rise in prevalence of the infection and large population. The adenovirus diagnostic market is expected to gain traction in Africa, where a large number of children infected with adenovirus are underserved.
Request TOC @https://www.transparencymarketresearch.com/sample/sample.php?flag=T&rep_id=1456
Key players in the global adenovirus diagnostics market include Agilent Technologies, Beckman Coulter/Danaher, Becton, Dickinson and Company, Affymetrix, Bio-Rad, Roche, Novartis AG, Gen-Probe, Qiagen, Abbott, DiaSorin, and bioMerieux.
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector - such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports strive to provide clients to serve their overall research requirement.
Contact Us
Transparency Market Research
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: [email protected]
Website: https://www.transparencymarketresearch.com
0 notes
Text
Adenovirus Diagnostics Market Size, Status and Forecast to 2026
Adenovirus is one of the DNA viruses that is considered to be the primary cause of fever illnesses, mostly among children. Individuals and children with chronic respiratory complications, cardiac disease, or weak immune systems are at higher risk of contracting adenovirus infection. However, most of the infections caused by adenovirus are not too severe. Adenovirus is a contagious infection, as it is an airborne disease and can spread from a person who is already infected to others through sneezing, cough, and close contact. Adenovirus can cause acute respiratory disease, pneumonia, epidemic keratoconjunctivitis, gastroenteritis, acute follicular conjunctivitis, and cystitis. Among children, pharyngitis fever and pharyngeal-conjunctival are the most common infection caused by adenovirus. A research conducted by the Barcelona Center for International Health Research and the University of Barcelona in 2016 indicated that adenovirus is the second most common respiratory virus that causes pneumonia. Few strains of adenoviruses can also spread through the infected person’s stool. Adenovirus can also spread through water such as swimming pools; however, this is not common. Infection caused by adenovirus can be usually diagnosed by molecular methods or detected with serology, polymerase chain reaction (PCR), viral culture, or through antigen detection. New technologies such as liposomes, chromatography, monoclonal antibodies, gel microdroplets, and flow cytometry are emerging as the methods of choice for the diagnosis of adenovirus infection.
Obtain Report Details @
https://www.transparencymarketresearch.com/adenovirus-diagnostic-market.html
Authentication of the infection caused by adenovirus is important to identify the most suitable treatment in order to establish a prediction and start a treatment procedure to control the infection by the application of accurate antiviral agents. This, in turn, is expected to boost the growth of the global adenovirus diagnostics market. In the recent past, adenovirus infections were diagnosed by cell culture, as the virus replicates efficiently in cell cultures. Detection and amplification of adenovirus DNA by different PCR methods are becoming the most widely accepted laboratory procedures for the detection of adenovirus infection. The swift detection and quantitation of adenovirus DNA, especially by techniques such as PCR, are expected to support the identification and treatment monitoring of adenovirus infections, particularly in immune-compromised patients. This, in turn, is expected to boost the growth of the global adenovirus diagnostic market during the forecast period. Moreover, increase in investment in research and development and technological advancements in the diagnosis of acute respiratory infections of viral etiology are expected to boost adenovirus diagnostics market growth.
Request Report Brochure @
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=1456
The global adenovirus diagnostics market can be segmented based on diagnostic method and end-user. In terms of diagnostic method, the adenovirus diagnostics market can be categorized into viral culture, polymerase chain reaction assays and antigen-based assays, monoclonal antibodies, liposomes and flow cytometry, chromatography, and gel microdroplets. Based on end-user, the global adenovirus diagnostics market can be divided into hospitals, private diagnostic laboratories, small clinics, and public health laboratories.
In terms of region, the global adenovirus diagnostics market can be segmented into five major regions: North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. The adenovirus diagnostic market in North America is expected to gain significant traction, owing to increase in public health concerns regarding various infectious diseases such as pneumonia, which is associated with adenovirus infection and reimbursement for adenovirus diagnostics. Moreover, there are a number of hospitals and private diagnostics laboratories in the U.S. and Canada that offer a broad portfolio of tests to determine the presence of adenovirus. Europe held significant share of the global adenovirus diagnostics market. The adenovirus diagnostics market in Asia Pacific is expected to grow at a rapid pace due to rise in prevalence of the infection and large population. The adenovirus diagnostic market is expected to gain traction in Africa, where a large number of children infected with adenovirus are underserved.
Enquiry for Discount on this Report @
https://www.transparencymarketresearch.com/sample/sample.php?flag=D&rep_id=1456
About Us
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Contact Us
Transparency Market Research
State Tower,
90 State Street, Suite 700
Albany, NY 12207
United States
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: [email protected]
Website: www.transparencymarketresearch.com
0 notes
Text
Most Common Signs Of Painful Bladder Syndrome!
The kidneys make urine, which is a liquid through which squanders from the body including urea are dispensed with from the body. There are two kidneys on the privilege and left side, which make urine, and pass it down to the bladder through tubes known as ureter. The bladder goes about as a supply of the urine that is shaped in the kidneys. It is put away incidentally there before being discharged out of the body through the urethra. The urinary bladder is an exceptionally solid organ and has a rich connective tissue.
Interstitial cystitis (IC) or difficult bladder disorder (PBS) is an extremely normal condition, which influences females more than guys. While the correct etiology isn't known, it could be age-related and furthermore way of life related. Individuals who are accustomed to controlling the inclination to pass urine are exceedingly liable to build up this condition. The straightforward rationale is that there is extra weight on the bladder from the urine that is contained for longer time frame. All things considered, the solid divider stretches and starts to feel pushed.
At the point when this propensity proceeds over some stretch of time, the bladder divider may wind up aggravated or aroused or even scarred in serious cases. There is no part of microorganisms in this condition, and anti-microbials are of no assistance in dealing with this condition (however the name cystitis as a rule shows contamination). These words are by Dr. Vijay Agrawal, he has done MCh, MS - Urology. He is an experienced Urologist in Athwagate, Surat. Dr. Vijay Agrawal is one of the best Urologists in Nanpura Surat.He has been a successful Urologist for the last 30 years. He has done MCh, MS - Urology. He is currently practising at Vijay Urological Hospital in Athwagate, Surat.
The accompanying side effects are viewed because of this steady bothering and irritation.
One may experience the ill effects of agony and weight in the bladder as it keeps on gathering increasingly urine.
This weight in the bladder likewise puts weight on the encompassing tissues in the stomach area including the pelvis, urethra, stomach organs, uterus, and so forth.
Ladies may encounter torment in the vaginal tract including vulva and behind the vagina.
Men may encounter torment in the territory of the scrotum, balls, prostate, and penis.
There is an expanded inclination to urinate, which might be as much as 9 to 10 times each day. As the condition advances, there could be more visits, upwards of 40 to 50 visits per day.
This propensity and desire to urinate increments amid evening.
For ladies, this desire to urinate and different side effects including torment are more terrible amid feminine cycle.
There could be torment amid intercourse for the two people.
There could be torment even something else, which can go from a mellow dull yearn to a piercing torment.
At a basic level, this consistent weight prompts pinpoint dying (glomerulations) and now and again even ulcers in the bladder divider.
There is no conclusive treatment for IC or PBS.

0 notes