#genetic engineering
Text


Merry Christmas here's your gays
#tf2#science party#team fortress 2#art-chi#engiemedic#tf2 medic#engineer x medic#tf2 engineer#medic x engineer#medicengie#genetic engineering#german engineering#genetic engineering tf2#german engineering tf2#Christmas#Archimedes#Digital art#gay
687 notes
·
View notes
Text



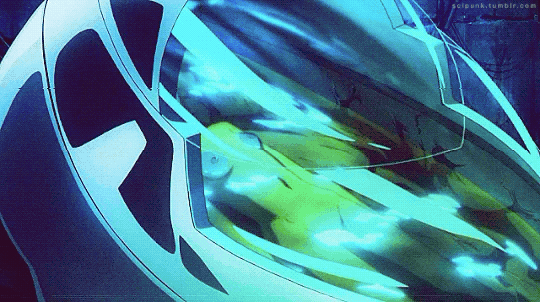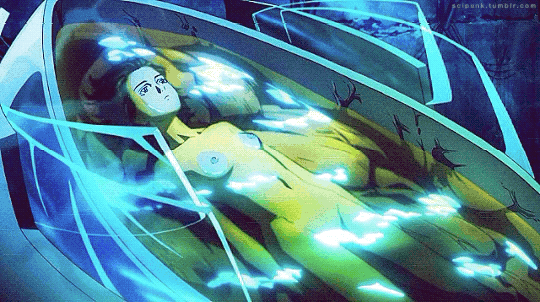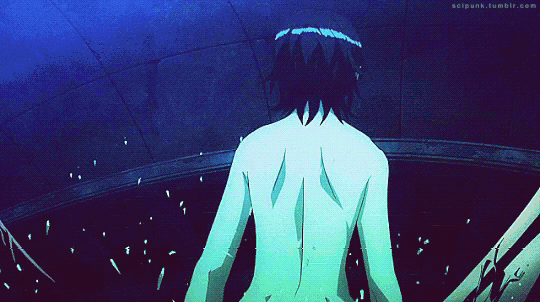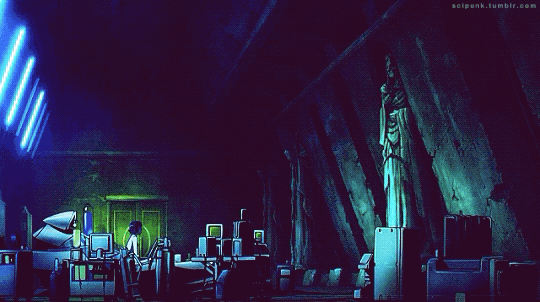

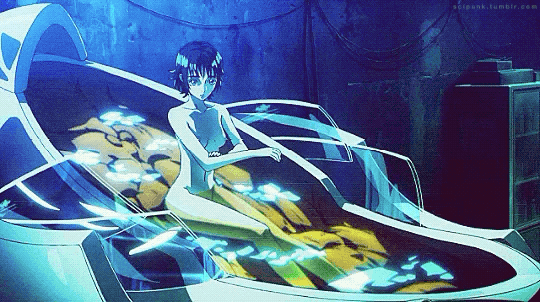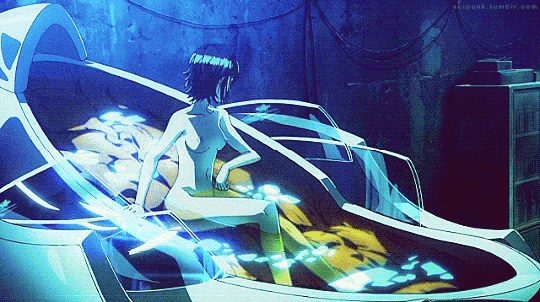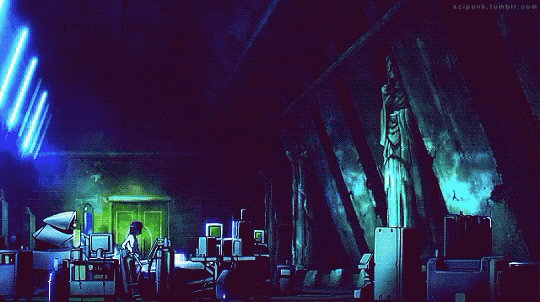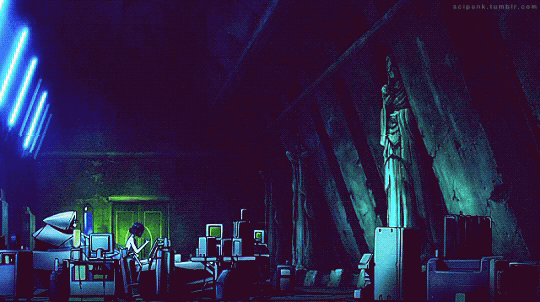
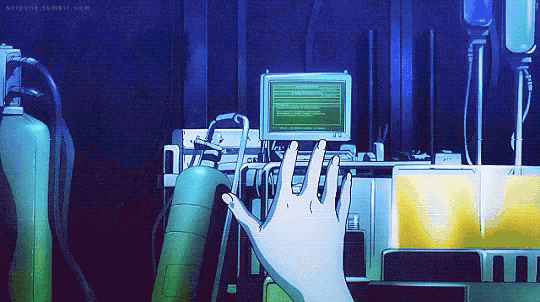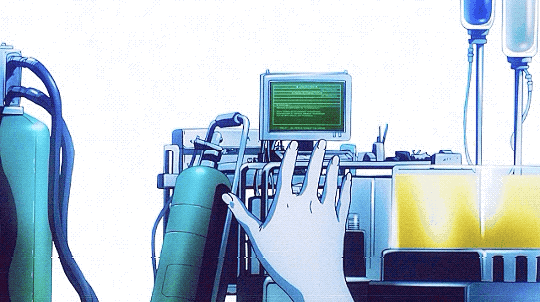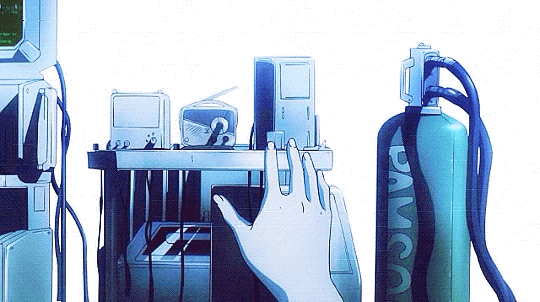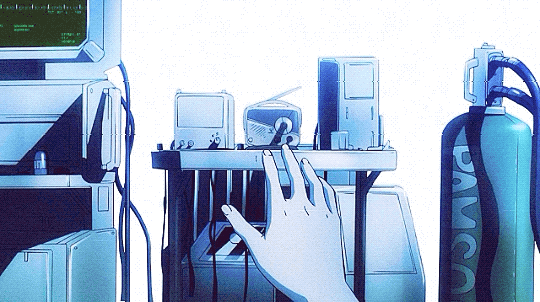


Mardock Scramble: The First Compression (2010)
#mardock scramble#anime#cyberpunk aesthetic#scifi#cyberpunk anime#cybernetics#japanese animation#computers#cyberpunk#gif#genetic engineering#high tech#human experimentation#gifset#neo noir#scifi anime#anime edit#anime gif
292 notes
·
View notes
Text

Plays More Gun, romantically
#tf2#team fortress 2#science party#engineer x medic#medic x engineer#genetic engineering#tf2 engineer#tf2 medic
867 notes
·
View notes
Text


Cowboys….
#tf2#team fortress 2#engiemedic#science party#genetic engineering#tf2 medic#tf2 engineer#tf2 fanart#0art0#yo what if dell took medic around texas tho…
418 notes
·
View notes
Text
"A company in France has developed genetically-enhanced houseplants that remove 30 times more indoor air pollutants than your normal ficus.
Paint, treated wood, household cleaners, insulation, unseen mold—there is a shopping list of things that can fill the air you breathe in your home with VOCs or volatile organic compounds. These include formaldehyde and other airborne substances that can cause inflammation and irritation in the body.
The best way to tackle this little-discussed private health problem is by keeping good outdoor airflow into your living spaces, but in the dog days of summer or the depths of a Maine winter, that might not be possible.
Houseplants can remove these pollutants from the air, and so the company Neoplants decided to make simple alterations to these species’ genetic makeup to supercharge this cleaning ability.
In particular, houseplants’ natural ability to absorb pollutants like formaldehyde relies on them storing them as toxins to be excreted later.
French scientists and Neoplants’ co-founders Lionel Mora and Patrick Torbey engineered a houseplant to convert them instead to plant matter. They also took aim at the natural microbiome of houseplants to enhance their ability to absorb and process VOCs as well.
The company’s first offering—the Neo P1—is a Devil’s ivy plant that sits on a custom-designed tall stand that both maximizes its air-cleaning properties and allows it to be watered far less often.
Initial testing, conducted by the Ecole Mines-Telecom of Lille University, shows that if you do choose to shell out the $179 for the Neo P1, it’s as if you were buying 30 houseplants. Of course, if you went for the budget route of 30 houseplants, you’d have to water them all.
The founders pointed out in an interview done with Forbes last year that once they settled on the species and fixed the winning genetic phenotype, the next part of the process was just raising plants, the same activity done in every nursery and florist in every town in Europe."
Deliveries for the P1 are estimated for August 2024.
-via Good News Network, November 6, 2023
--
Note: I'm not a plant biologist, but if this works the way the company's white paper says it does, holy genetic engineering, Batman.
(Would love to hear thoughts from anyone who is a plant biologist or other relevant field!)
#plant biology#superplant#pollution#indoor plants#plantblr#house plants#plantlife#hope posting#solarpunk#small business#genetic engineering#genetics#molecular biology#microbiome#respiratory health#france#ivy
554 notes
·
View notes
Text
Researchers have genetically engineered a marine microorganism to break down plastic in salt water. Specifically, the modified organism can break down polyethylene terephthalate (PET), a plastic used in everything from water bottles to clothing that is a significant contributor to microplastic pollution in oceans.
"This is exciting because we need to address plastic pollution in marine environments," says Nathan Crook, corresponding author of a paper on the work and an assistant professor of chemical and biomolecular engineering at North Carolina State University.
"One option is to pull the plastic out of the water and put it in a landfill, but that poses challenges of its own. It would be better if we could break these plastics down into products that can be re-used. For that to work, you need an inexpensive way to break the plastic down. Our work here is a big step in that direction."
To address this challenge, the researchers worked with two species of bacteria. The first bacterium, Vibrio natriegens, thrives in saltwater and is remarkable—in part—because it reproduces very quickly. The second bacterium, Ideonella sakaiensis, is remarkable because it produces enzymes that allow it to break down PET and eat it.
Continue Reading
420 notes
·
View notes
Text
#vegan leather#plastic pollution#plastics#pleather#leather alternatives#leather#environmentalism#good news#science#environment#bacteria#microbiology#genetic engineering
152 notes
·
View notes
Text
“What are you looking at so intently?” says Engie like any good Texan boy would, all fluttering eyelashes and blushy grins despite the fact his eyes are behind half a centimeter of tinted glass. Medic puffs out his lips into a little pucker, then smooths back out, and Engie glints with satisfaction at just the lightest dusting of pink that rises to his cheeks.
He makes sure to be just a little extra slow and sultry when he leans over and plucks a beer from the case by his recliner-chair. “See somethin’ ya like?” By which he means: you're staring for a good reason, please, god, aren't you? But he’s too respectful and upstanding to say that, though he considers himself certainly talented at this whole implications thing, and the deep mauve Medic turns when he tosses just a slightly inflected look in his direction indicates he must get the general idea.
“Well—hoo, well.” He spends two seconds frankly adorably stumbling through his words and then that gloved hand darts out and there’s rubber around Engie’s wrist, two long, thick fingers barely touching thumb-to-pointer in a little ring. “I was—er…”
He rescinds the hand, temples his fingers under his chin, caught between sitting and standing and settling in a weird in-between as Engie watches, fascinated. “I was just… thinking… about your heart. And your lungs.” He tilts his head. “And, in a small measure, your brain, I suppose. Er, imagining your breathing, and your circulation, and the oxygen flowing in and out of your limbs, and… so on. Drawing your veins in my mind and, and such.”
… Huh. Maybe he misread the situation.
And he keeps just stumbling through, pushing up his glasses every few seconds, still perched on his heels with his arms wrapped around his knees. “You’re a—private man. I have not seen much of you besides what I have been able to—have the company require,” and his voice pushes up a few notes on the last word, and well he’ll be damned if it’s not the cutest thing he’s ever seen, “but I… think about you.”
He lowers his voice just a little for this one, reclining down onto his back. “Oh, you do.”
“You have a very impressive set of lungs. Even for all the, er, damage." Engie frowns and Medic puts his hands up. "That's a compliment."
"Uh-huh."
"And your heart is a perfect specimen. Strong, and healthy… pulsing with vitality," and his eyes bug out just a little on the last line. "I was almost upset to have to cut it out of you… until, well…"
Ain't that interesting. "Until?"
He smiles sheepishly, wringing his hands together with a frankly disgusting sound of rubber against rubber. “… I may or may not have kept your original heart in a jar. With my other personal possessions.”
He mentally re-catalogues everything of interest in the lab, mentally travels to Medic’s big mahogany former-bookshelf, stacked top-to-bottom with preserved organs in jars, and sees a lot of hearts. A lot of hearts. But, then again, his mind is drawn back to a smallish mason jar near the front, suspiciously unlabeled, amidst rows and rows of perfectly organized bits and pieces.
Yeah, sure.
He's sure Medic is approaching this more from scientific curiosity than any particular angle he'd prefer, but heat's rising to his face before he knows it and frankly he doesn't really feel like putting in the effort to quell it.
And just to press the envelope that tiny bit further, he ventures: "Well I'll be darned if that's not the most romantic thing I've ever heard."
Medic turns the color of his tie. Engineer's sure he follows suit.
#hi science party post for the first time in 16 years youre all welcome (visibly frothing at the mouth)#tf2 engineer#tf2 medic#science party#bird brains#genetic engineering#god ive spent so much time away from these eggheads i dont even remember which ship tag i normally use#tf2#bungus snippets#engiemedic
158 notes
·
View notes
Text
i adore in fics when people find out about julian’s enhancements and then julian lets down his guard and people can see an actual a change, like suddenly there’s something unfamiliar about him? something much deeper and mature? not because everything about julian before the revelation has been a SHAM or anything, i just like the idea of all these intelligent people having totally underestimated julian, the young, naive doctor, when really julian’s been one step ahead of them the whole time, letting them see him a certain way. the concept of others finally getting to see they had the wrong idea, especially back when they sort of saw julian as somewhat less than, the whole time. it’s about that moment of oh. he’s been the smartest guy in the room the whole time. them suddenly not seeing naivety, but seeing power. that. i like that.
#i guess this is another moment of me kinning julian a little too hard#i would love a moment like that#like oh. we had no idea did we#only i’m too much of a) an oversharer and b) a dumbass#julian bashir#star trek ds9#alexander siddig#star trek deep space nine#st: ds9#doctor bashir i presume#augment#star trek deep space 9#genetic engineering#star trek#ds9#trekkies#fanfiction#doctor bashir#gene roddenberry#garashir#fanfic#headcanons
152 notes
·
View notes
Text
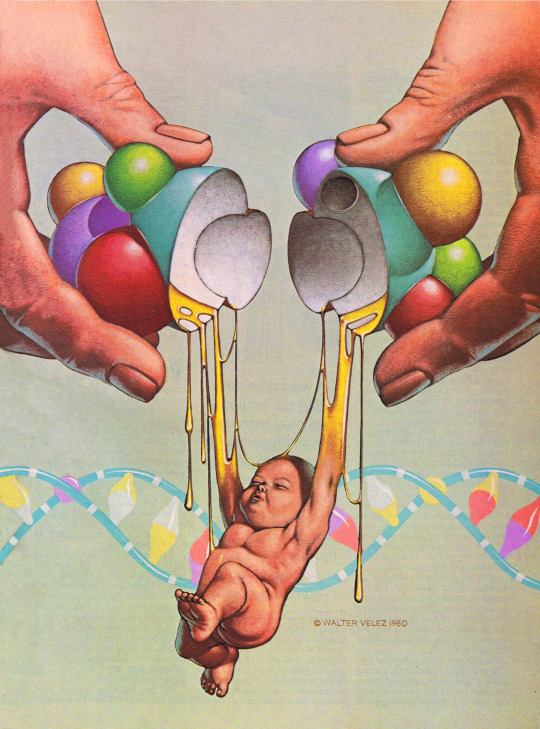
Walter Velez (1939-2018), ''Future Life'', #25, March 1981
Source
164 notes
·
View notes
Text






Them your honor
#tf2#science party#team fortress 2#art-chi#engiemedic#tf2 medic#engineer x medic#tf2 engineer#medic x engineer#medicengie#genetic engineering#german engineering tf2#german engineering#genetic engineering tf2
807 notes
·
View notes
Text

474 notes
·
View notes
Link
Through the advent of CRISPR-Cas12, scientists have managed to unlock a new world of gene editing and reprogramming, with important applications for diagnostics, therapeutics, agricultural science, and more. However, their bacterial origin may make them difficult to work with as researchers begin to explore the potential of human genome editing. A new study led by researchers from MIT found thousands of new RNA-guided enzymes known as Fanzors prevalent among eukaryotic organisms that show great promise as novel tools for mammalian genome editing.
RNA-programmable DNA nucleases are essential for the function of prokaryotes in the defense and proliferation of mobile elements. Such nucleases include the likes of CRISPR argonauts, as well as the nucleases that comprise the OMEGA systems (Obligate Mobile Element Guided Activity), which includes nucleases such as IscB, IsrB, and IshB, among others. Of these, one nuclease, the TnpB gene, contains a nuclease domain similar to the one found in the Cas12 gene, suggesting an evolutionary relationship between the two. This is further backed up by phylogenetic analysis conducted on the two domains, which indicates that different subtypes of Cas12 originated independently from different groups of TnpB enzymes. Furthermore, biochemical and cellular experiments have demonstrated that the TnpB-Omegas complex is an RNA-guided and programmable DNA endonuclease.
Continue Reading
79 notes
·
View notes
Text



Doodles with the time i have between commissions and course work B]
#tf2#team fortress 2#tf2 engineer#tf2 medic#engiemedic#science party#genetic engineering#tf2 fanart#0art0
259 notes
·
View notes
Text
"Scientists have created mice with two biological fathers by generating eggs from male cells, a development that opens up radical new possibilities for reproduction.
The advance could ultimately pave the way for treatments for severe forms of infertility, as well as raising the tantalising prospect of same-sex couples being able to have a biological child together in the future.
“This is the first case of making robust mammal oocytes [a.k.a. egg cells] from male cells,” said Katsuhiko Hayashi, who led the work at Kyushu University in Japan and is internationally renowned as a pioneer in the field of lab-grown eggs and sperm.
Hayashi, who presented the development at the Third International Summit on Human Genome Editing at the Francis Crick Institute in London on Wednesday, predicts that it will be technically possible to create a viable human egg from a male skin cell within a decade. Others suggested this timeline was optimistic given that scientists are yet to create viable lab-grown human eggs from female cells.
Previously scientists have created mice that technically had two biological fathers through a chain of elaborate steps, including genetic engineering. However, this is the first time viable eggs have been cultivated from male cells and marks a significant advance. Hayashi’s team is now attempting to replicate this achievement with human cells, although there would be significant hurdles for the use of lab-grown eggs for clinical purposes, including establishing their safety.
“Purely in terms of technology, it will be possible [in humans] even in 10 years,” he said, adding that he personally would be in favour of the technology being used clinically to allow two men to have a baby if it were shown to be safe.
“I don’t know whether they’ll be available for reproduction,” he said. “That is not a question just for the scientific programme, but also for [society].”
The technique could also be applied to treat severe forms of infertility, including women with Turner’s syndrome, in whom one copy of the X chromosome is missing or partly missing, and Hayashi said this application was the primary motivation for the research.
Others suggested that it could prove challenging to translate the technique to human cells. Human cells require much longer periods of cultivation to produce a mature egg, which can increase the risk of cells acquiring unwanted genetic changes.
Prof George Daley, the dean of Harvard Medical School, described the work as “fascinating”, but added that other research had indicated that creating lab-grown gametes from human cells was more challenging than for mouse cells. “We still don’t understand enough of the unique biology of human gametogenesis to reproduce Hayashi’s provocative work in mice,” he said.
Study Methods
The study, which has been submitted for publication in a leading journal, relied on a sequence of intricate steps to transform a skin cell, carrying the male XY chromosome combination, into an egg, with the female XX version.
Male skin cells were reprogrammed into a stem cell-like state to create so-called induced pluripotent stem (iPS) cells. The Y-chromosome of these cells was then deleted and replaced by an X chromosome “borrowed” from another cell to produce iPS cells with two identical X chromosomes.
“The trick of this, the biggest trick, is the duplication of the X chromosome,” said Hayashi. “We really tried to establish a system to duplicate the X chromosome.”
Finally, the cells were cultivated in an ovary organoid, a culture system designed to replicate the conditions inside a mouse ovary. When the eggs were fertilised with normal sperm, the scientists obtained about 600 embryos, which were implanted into surrogate mice, resulting in the birth of seven mouse pups. The efficiency of about 1% was lower [although not THAT much lower] than the efficiency achieved with normal female-derived eggs, where about 5% of embryos went on to produce a live birth.
The baby mice appeared healthy, had a normal lifespan, and went on to have offspring as adults. “They look OK, they look to be growing normally, they become fathers,” said Hayashi.
Going Further
He and colleagues are now attempting to replicate the creation of lab-grown eggs using human cells.
Prof Amander Clark, who works on lab-grown gametes at the University of California Los Angeles, said that translating the work into human cells would be a “huge leap”, because scientists are yet to create lab-grown human eggs from female cells.
Scientists have created the precursors of human eggs, but until now the cells have stopped developing before the point of meiosis, a critical step of cell division that is required in the development of mature eggs and sperm. “We’re poised at this bottleneck at the moment,” she said. “The next steps are an engineering challenge. But getting through that could be 10 years or 20 years.”
-via The Guardian (US), 3/8/23
#genetics#gene editing#genetic engineering#reproductive care#infertility#infertility cw#ivf#science and technology#lgbtq#oocytes#gametes#turner syndrome#queer parenting#good news#hope
213 notes
·
View notes
Text
Scientists in China have synthesized spider silk from genetically modified silkworms, producing fibers six times tougher than the Kevlar used in bulletproof vests.
The study, published September 20 in the journal Matter, is the first to successfully produce full-length spider silk proteins using silkworms. The findings demonstrate a technique that could be used to manufacture an environmentally friendly alternative to synthetic commercial fibers such as nylon.
"Silkworm silk is presently the only animal silk fiber commercialized on a large scale, with well-established rearing techniques," said Mi. "Consequently, employing genetically modified silkworms to produce spider silk fiber enables low-cost, large-scale commercialization."
Scientists have eyed spider silk as an enticingly sustainable alternative to synthetic fibers, which can release harmful microplastics into the environment and are often produced from fossil fuels that generate greenhouse gas emissions. But turning to nature for alternatives isn't without challenges.
Continue Reading
317 notes
·
View notes