Photo

A product crystallized in these nice needles in s small vial.
We planned to obtain an X ray diffraction to know an exact structure of this special molecule, unfortunately these needles are too thin for an X ray measurement.
177 notes
·
View notes
Photo

Just a snapshot from the fume hood as it looks like during a normal day.
159 notes
·
View notes
Photo

Preparation of an oxygen saturated solution in a graduated cylinder.
It is often painful to know the reason of side reactions or even get clues about how a reaction proceeds. In this case the reaction was performed in an oxygen saturated atmosphere to know that a side product that formed during the transformation is a result of an oxidation or something else is happening.
79 notes
·
View notes
Photo


Barbier-type allylation of aldehydes with allylic bromide and tin(II) chloride in presence of potassium iodide and ammonium chloride in water.
During the reaction tin(II) iodide forms what is visible from the color change of the reaction. Tin(II) iodide is a bight orange salt that is not really soluble in water in the presence of ammonium ions.
The Barbier reaction is an organometallic reaction between an alkyl halide (chloride, bromide, iodide), a carbonyl group and a metal. The reaction can be performed using magnesium, aluminium, zinc, indium, tin, samarium, barium or their salts. The reaction product is a primary, secondary or tertiary alcohol. The reaction is similar to the Grignard reaction but the crucial difference is that the organometallic species in the Barbier reaction is generated in situ, whereas a Grignard reagent is prepared separately before addition of the carbonyl compound.
The Barbier reaction is named after Victor Grignard's teacher Philippe Barbier.
97 notes
·
View notes
Photo


Performing a photochemical transformation using white light and the slow addition of a photocatalyst.
On left there is a reaction that is stirred on a magnetic stirrer and kept under a slight over pressure of nitrogen gas to keep the reaction oxygen free. From the other side a quite old, but still perfectly working syringe pump is used to add a reagent to the reaction with a really low speed though a rubber septa.
This technique is frequently used when low concentration matters and it is critical to keep at least one of reactants on a low but constant concentration.
110 notes
·
View notes
Photo


When we ran out of space and magnetic stirrers in the fume hood quite crowded oil baths are in operation.
In this case, total 6 reaction is performed on quite wide variety of scales and using different techniques. From standard round flasks, to pressure tubes a lot chemistry is happening over there!
59 notes
·
View notes
Photo


Recrystallizing a raw product using an infra lamp as a heat source.
It looks great and it also works well. Instead of a heat gun or an oil bath, we often use infra lamps as heat source during the recrystallization of various products. It is a safe and easy method for heating a flask.
2K notes
·
View notes
Photo

The lab ran out of balloons that we often use to keep reactions under nitrogen atmosphere and a colleague had a a desperate attempt to use a nitrile glove instead of a balloon.
I think that anyone is able to decide whether or not it has been successful...
55 notes
·
View notes
Photo



Melting some silver.
It was melted in a ceramic crucible with an oxygene/methane torch, under circa 1h. The silver was liquified under a layer of borax (it prevents the oxidation of the molten silver).
After all have melted we just dropped it in a bucket of water and took out this shiny lump of this beautiful metal.
444 notes
·
View notes
Photo


Reduction using some freshly prepared sodium naphthalenide.
It is also known as sodium naphthalide, is an organic salt with the chemical formula Na+C10H8−. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry.

The alkali metal naphthalenides are prepared by stirring the metal with naphthalene in an ethereal solvent, usually as tetrahydrofuran or dimethoxyethane. The resulting salt is dark green as seen on the bottom of the first picture and on the above seen gif.
133 notes
·
View notes
Photo

Filtration of a reaction mixture though a pad of silica gel.
It is a well working technique for the purification of different reaction mixtures. If a highly polar, non elutable side product if formed during the reaction, it will stay on the silica gel, and only the “washable” things will go through. It you are lucky enough, no other purification is needed and only an evaporation is required to obtain a pure compound.
75 notes
·
View notes
Photo
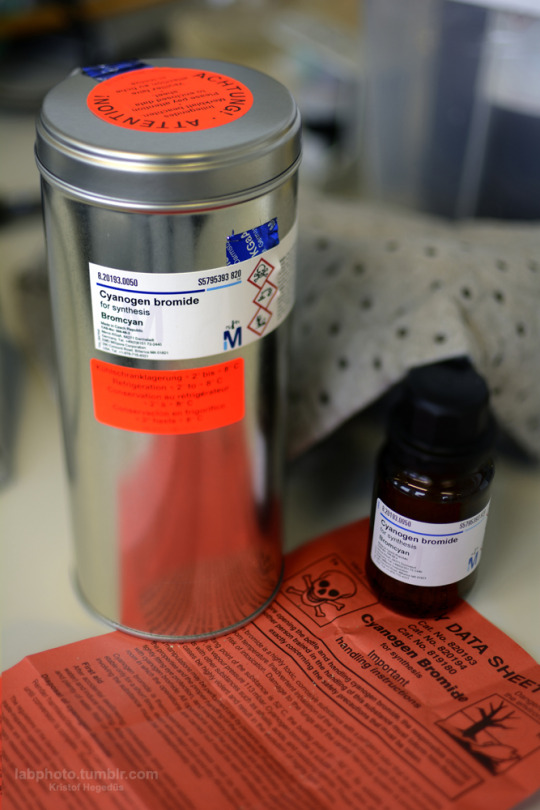
A freshly arrived can of cyanogen bromide.
Cyanogen bromide is the inorganic compound with the formula BrCN. It is a colorless solid that is widely used in organic chemistry.
Some interesting data from the book “toxic industrial compounds” directly from Russia, written in 1957:
For cyanogen bromide it says:
Breathing 0,006 mg/l concentration of BrCN has a strongly irritating effect if inhaled for 10 minutes
0,4 mg/l death if inhaled for 10 minutes.
65 notes
·
View notes
Photo



Working with tert-butyllithium.
This one is a quite useful and well known chemical. It spontaneously ignites on air. tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon acids, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory.
tert-Butyllithium is a pyrophoric substance, meaning that it easily catches fire on exposure to air. While it is possible to work with this compound using cannula transfer, traces of tert-butyllithium at the tip of the needle or cannula may catch fire and clog the cannula with lithium salts. Here we transferred it with a plastic syringe under N2 atmosphere.
Serious laboratory accidents involving tert-butyllithium have occurred, so be careful with this compound!
258 notes
·
View notes
Photo

Screening different reaction conditions using a catalystin a special reaction. Each vial contains a different amount/conentration of reactant or another solvent.
Often to find the perfect conditions for an organic chemical transformation is a long and complex task. It could take dozens reactions and LOT time to get good result. It is a hard thing to prepare a compound, another hard task is to prepare it in a pure form and a harder quest is to make it on an efficient way.
103 notes
·
View notes
Photo



Distilling a reaction mixture using a short path distillation apparatus.
The short path distillation apparatus is used when we have to distill heat sensitive compounds that may decompose under prolonged heating. In this case if the boiling point is reached, the hot steam of the compound should only travel a short path til it is condensed down on the cold finger.
69 notes
·
View notes
Photo

Just a deep red reaction mixture that slowly separates from the water phase in a separating funnel.
In this case a dichloromethane solution was washed with brine and due the similar density of the saturated brine to the dichloromethane (1.33 g/cm3) these type of extractions are hard to separate. If this happens a mild dilution of the brine, or the organic phase could help. If the density of the two solution differs a bit, rapid phase separation will happen.
326 notes
·
View notes
Photo


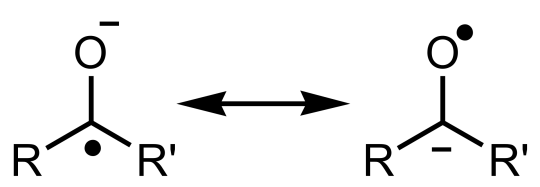
Making some fresh, absolute tetrahydrofuran (THF) with benzophenone and sodium. The diphenylketyl-sodium has this beautiful deep blue color what only appears if no water, oxygen, CO2 or anything bad is left in the solution.
This treatment is used for removing traces of water from the solution.
The benzophenone ketyl is an anion radical that contains a group Ph2C−O•. It is the product of the 1-electron reduction of a ketone.
187 notes
·
View notes