#bmj open author guidelines
Text
Os 21 Principais Periódicos Médicos de Acesso Aberto Que Os Especialistas em Observação de Tecnologia Devem Aproveitar
Introdução : Acompanhar o cenário tecnológico em constante evolução na área médica pode ser um desafio. Se você é um especialista em observação de tecnologia, precisa ser capaz de identificar rapidamente as tendências emergentes e tomar decisões sensatas com base nelas. Mas como você pode [...] https://is.gd/Gg0jGx

#business #communication #data #education #ict #information #intelligence #technology - Created by David Donisa from Academypedia.info
#a revista médica britânica é confiável#acesso aberto híbrido#acesso aberto verde#banco de dados de acesso aberto#bmj abrir enviar#bmj open author guidelines#bmj qualidade aberta#bmj submissão aberta#bronze acesso aberto#ciência aberta#classificação de revistas#desvantagens de acesso aberto#diamante acesso aberto#diretório de livros de acesso aberto#diretório doaj de periódicos de acesso aberto#doab#etapas do processo de revisão por pares#exemplos de acesso aberto#exemplos de ameaças em uma análise swot#exemplos de oportunidades de análise swot para alunos#fator de impacto aberto bmj#fator de impacto bmj#fator de impacto da revista de psicologia#fator de impacto de qualidade e segurança bmj#fator de impacto de relatórios de casos bmj#índice h vs fator de impacto#jornais e editores predatórios#jornal de acesso aberto atrasado#lista de revistas médicas de acesso aberto#melhores revistas médicas de acesso aberto
0 notes
Text
Top 21 Open Access Medical Journals That Technology Watch Experts Should Leverage
Introduction : Keeping up with the ever-evolving technology landscape in the medical field can be a challenge. If you’re a technology watch expert, you need to be able to quickly identify emerging trends and make sound decisions based on them. But how can you do that ? This a [...] https://is.gd/f0qghy

#business #communication #data #education #ict #information #intelligence #technology - Created by David Donisa from Academypedia.info
#are open access journals bad#best open access medical journals#bmj case reports#bmj case reports impact factor#bmj impact factor#bmj open author guidelines#bmj open impact factor#bmj open quality#bmj open submission#bmj open submit#bmj quality and safety impact factor#bronze open access#delayed open access journal#diamond open access#directory of open access books#doab#doaj directory of open access journal#examples of threats in a swot analysis#free open access medical journals#gold open access#green open access#h-index vs impact factor#hybrid open access#is scientific reports a good journal#is the british medical journal reliable#journal ranking#list of open access medical journals#open access database#open access disadvantages#open access examples
0 notes
Text
Sitting for more than three hours a day can cut two years off a person's life expectancy, even if he or she exercises regularly, a new study finds. Watching TV for more than two hours a day can shorten life expectancy even further, by another 1.4 years.
The findings suggest that when it comes to gleaning health benefits from physical activity, it may not be enough just to get the recommended amount of daily exercise — the government advises about a half-hour of moderate activity a day for adults. But what about the other 23.5 hours of every day? Researchers say it's important not to spend it sedentary or sitting.
In the same way that both pushing the gas and hitting the brake can adjust the speed of your car, researchers say that physical activity and sedentary behavior independently affect your health and life expectancy. Whether "you're physically active and meet the exercise guidelines, or if you're not active," says Peter Katzmarzyk, professor of epidemiology at Pennington Biomedical Research Center and lead author of the new paper published in the online journal BMJ Open, "sitting is bad."
1 note
·
View note
Text
How to Create Guest Post Guidelines for Your Blog
Tolerating visitor posts ought to be significantly less demanding than it is.
In case you're searching for an approach to grow the measure of substance on your site while incorporating connections and tapping with the specialist/crowds of other individuals, opening your site up to visitor posts is a brilliant move.
Destinations like Moz, Torque and even Medium have constructed their organizations on outside commitments, and when the procedure works, it works extremely well.
All things considered, this isn't a system to waltz into aimlessly or without arrangement.
Ideally, you'd just get entries from qualified individuals who know your blog, regard your gathering of people and compose like prize-winning creators.
As a general rule, you'll likely be immersed with pitches from a wide range of interesting people from everywhere throughout the web. Without investing energy forthright planning to subdue the tide, you'll end up investing hours dealing with terrible and superfluous substance before finding those pearls deserving of distributing.

Be that as it may, it shouldn't be that way.
Visitor post rules are your first line of protection against unimportant or inadequately composed substance, and in this post, I'll demonstrate you all that you have to consider and incorporate into your rules to help stem the tide and spare you long stretches of checking on pitches and altering posts (and trust me, you WILL be altering posts, regardless of how great your rules might be).
For example, the service Customwriting is a relevant essay writing platform. The big amount of students pay on https://customwriting.com/admissions. My friends say that writers always follow all requirements.
The Essential Components of Effective Guest Post Guidelines
I'm going to methodicallly separate a coherent stream for your rules that will lead potential benefactors through all that they need to think about getting content on your site.
1. Opener
While it may appear negligible duplicate, an opening message is your opportunity to affirm that indeed, you acknowledge visitor commitments, yet no, it is anything but a free-for-all. This is your opportunity to clarify:
Your identity: While you'd trust that whoever is reaching you would as of now comprehend the kind of site/business you are, this isn't generally the situation. This opening section from The Humanist's visitor post rules clarifies what sort of distribution they are:
Who you achieve: Help visitor notices comprehend the group of onlookers that you achieve, rapidly sharing socioeconomics, psychographics, work titles and increasingly that will portray who the substance is planned for. This can enable weed to out unessential commitments, as well as draw in the correct sorts of creators.
Your objectives/vision: What does your organization/blog really represent? What is the message you need to send? What would you like to accomplish? The majority of this is typically established in settling the torment purposes of a client, making the past point even more essential.
2. Who can compose for us?
The following segment you need to spread out is who is welcome to distribute on your blog. In case you're available to all commitments, say as much—yet this may not be the situation. For instance, you might need to determine that you just take blog entries from…
Non-contenders
Those specifically enterprises or jobs
Perceived specialists
For instance, these rules from Student BMJ promptly avoid commitments from the individuals who don't meet strict criteria:
You'll likewise need to make reference to regardless of whether you pay for visitor content, and provided that this is true, it may make reference to how much. While referencing you pay out $200/post (or whatever the figure might be) may pull in some obnoxious sorts hoping to make a fast buck, it can likewise go about as a motivating force for more legitimate creators to invest energy making a bit of substance for your site.
3. sorts of substance acknowledged
Just intrigued by assessment pieces or how-to articles? Not intrigued by local promotions or anything deals centered? It's essential to determine the sorts of substance you'll acknowledge, including the points you're willing to distribute on.
Determine regardless of whether you acknowledge syndicated/unimaginative posts (most destinations request a post be absolutely special).
Notice the sorts of posts you don't acknowledge (public statements, and so on.).
Feature and emphasize the points you acknowledge visitor posts on. Clarify that any posts outside of these points won't be considered.
Incorporate connects to tests of extremely effective posts on the site. This makes it a lot less demanding to know whether a pitch will be a fit.
This is a decent time to make reference to your distributing recurrence and how regularly new posts are pushed live. Setting this desire ahead of time will enable you to abstain from getting annoyed by creators who figured their post would be live inside the week.
Critical: Specify whether you acknowledge pitches, drafts or both. In the event that you need to pre-endorse pitches as opposed to searching through drafts, say as much. On the off chance that you'd preferably observe the whole composed post from the get-go, let authors know.
This example from the MarketingProfs' rules makes it evident that just particular sorts of posts will be acknowledged, at that point connects to instances of past posts that assistance an imminent visitor blurb comprehend what will be required.
1 note
·
View note
Photo
A study from the UIC College of Pharmacy found that it takes nearly three years for treatments approved through expedited FDA approval processes to be incorporated into the clinical guidelines doctors use to help determine treatment for their patients.
The truth about patient access and FDA fast-tracking
University of Illinois Chicago researchers studied 135 products that received U.S. Food and Drug Administration approval through orphan drug and expedited approval program designations. They found that it took nearly three years for these drugs and biologics to be incorporated into the clinical guidelines doctors use to help determine treatment for their patients.
The findings suggest that despite efforts to speed up the availability of these products, which are commonly expedited because of their potential to treat rare, serious or life-threatening conditions, the most direct guidance on patient access to the treatments is still delayed.
“This analysis provides evidence that expedited approval processes are only the first step to bring needed treatment options to patients who have rare diseases or have exhausted other options,” said study corresponding author Ryan Rodriguez, clinical associate professor of pharmacy practice at the UIC College of Pharmacy. “FDA approval is not the end of the story when it comes to patient access. We need to do a much better job of planning ahead so that clinicians are aware of and have confidence in administering newly available treatments much earlier.”
The findings are reported in the medical journal BMJ Open.
For their analysis, Rodriguez and his colleagues identified products with one or more expedited designations — priority review, accelerated approval, breakthrough therapy and/or fast track — approved from January 1992 to February 2020 using publicly available data from FDA databases. They excluded cancer-related products from their analysis. Using PubMed and guideline databases, the researchers looked for published guidance documents influencing U.S. practice that included each product.
Among the 135 non-oncological orphan products identified, the researchers found that 97% were designated priority review, 50% fast-track, 16% breakthrough therapy, and 14% accelerated approval. More than half (60%) received two or more designations.
While about 74% of products were included in a guidance document, the median time to inclusion was 2.87 years. Similarly, of the products not found in a guidance document, more than half (54%) had received “recent” approval, in 2018 or later.
The researchers found that products were more likely to be included in a guidance document if they had received two or more designations. Products were also more likely to be included in guidelines if they received fast-track designation compared to priority review designation.
The authors say these findings are particularly important considering that the annual number of orphan drug designations assigned by the FDA has increased over the last two decades. According to one study from 2018, the number has increased from roughly 60 in 2002 to 427 in 2017. The predicted mean annual growth rate for orphan products (11%) is more than double the predicted rate for non-orphan products (5%).
“It’s very likely that use of these designations will continue and even increase over the coming years, so it’s critical that we build parallel processes to reduce the access delays we may see today for this growing category of products,” Rodriguez said.
The authors suggest that the three-year delay may be attributed, in part, to the resource investment required for rigorous guideline development — a process they say can be streamlined. They also suggest that the delay from approval to guidance may be related to the limited scientific evidence available at the time of product approval but are quick to point out that this is surmountable.
“Recommendations for use of orphan drugs should be supported by robust evidence; such evidence may not be immediately available, and professional societies may deem full guidance production or revision unjustified. Nonetheless, statements regarding the limited evidence and recommendations for restricted, yet appropriate, use can still be useful to policymakers in justifying coverage decisions,” according to Rodriguez and his colleagues.
In conclusion, the authors write, “More timely development of guidance documents after approval of these products could encourage more rapid and appropriate uptake into practice and could be initiated when FDA approval is anticipated.”
Rodriguez said, “This data shows us there is a problem and that consequences fall on the patient — I hope researchers, regulators, industry and medical societies alike read our study and see that there is a great opportunity to get more treatment options to patients simply by considering actions that encourage appropriate inclusion of orphan and expedited program products in relevant guidelines.”
Co-authors of the BMJ Open article, “Time to inclusion in clinical guidance documents for non-oncological orphan drugs and biologics with expedited FDA designations: a retrospective survival analysis,” are Rachel Brunner and Samantha Spencer of UIC and Dima Qato of the University of Southern California.
The research was not funded by any public, commercial or nonprofit agency.
1 note
·
View note
Text
Long-Term CRYSVITA® ▼ (burosumab) Treatment Reduces the Burden of Disease in Adults With X-Linked Hypophosphataemia (XLH), a Rare Genetic Metabolic Bone Disease
New Post has been published on https://depression-md.com/long-term-crysvita-%e2%96%bc-burosumab-treatment-reduces-the-burden-of-disease-in-adults-with-x-linked-hypophosphataemia-xlh-a-rare-genetic-metabolic-bone-disease/
Long-Term CRYSVITA® ▼ (burosumab) Treatment Reduces the Burden of Disease in Adults With X-Linked Hypophosphataemia (XLH), a Rare Genetic Metabolic Bone Disease

TOKYO–(BUSINESS WIRE)–Kyowa Kirin Co., Ltd. (TSE:4151, Kyowa Kirin) today announced the publication of new data highlighting the sustained benefits of treatment with CRYSVITA® (burosumab) in adults with X-linked hypophosphataemia (XLH), a rare genetic metabolic bone disease. The data show that adults with XLH experience substantial pain, stiffness, fatigue and impairment in physical and ambulatory function. Treatment with CRYSVITA was associated with a significant improvement from baseline after 96 weeks.1
The data are from a randomised, double-blind, placebo-controlled, phase 3 study with an open-label extension to assess the efficacy and safety of CRYSVITA in adults with XLH.2 The study met its primary endpoint, showing a statistically significant effect in increasing serum phosphorus concentrations at 24 weeks, compared to placebo.3 After 24 weeks, all patients were switched to CRYSVITA treatment and data was collected on metabolic and biochemical markers, patient reported outcomes (PROs) and measures of mobility up to 96 weeks. This new publication focuses on the results from the PRO analysis and mobility scores.1
At week 96, the study showed statistically significant improvements in PROs, including the Western Ontario and the McMaster Universities Osteoarthritis Index (WOMAC), Brief Pain Inventory–Short Form (BPI-SF) and Brief Fatigue Inventory (BFI), compared to baseline.1 Statistically significant improvements in ambulatory function, measured by the 6-minute walk test (6MWT), were also seen at 96 weeks compared to baseline.1 Data previously published at 48 weeks also showed improvements in some PROs, including stiffness and pain, as well as fracture healing.3
Lead author Pr Karine Briot, Hôpital Cochin, Paris, France said: “The study highlights the many physical challenges faced by adult patients with XLH, including pain, stiffness, fatigue and difficulty walking or physical function. Burosumab treatment has previously been shown to improve phosphate homeostasis in adult XLH patients, compared to placebo. This new analysis suggests that, despite the long-term complications and physical impairment associated with XLH in adults, treatment with burosumab can also improve the physical function and quality of life of adults with XLH over the longer term.”
Tomohiro Sudo, Executive Officer, Head of Global Product Strategy Department of Kyowa Kirin, said: “Kyowa Kirin is committed to improving the lives of people with XLH and their families. One of our areas of focus is to generate new data that improve our understanding of how best to manage and treat XLH. These important new data highlight the many physical challenges that people living with XLH face every day, how their needs could be better met and how Kyowa Kirin is delivering on its purpose, to make people smile.”
The data were published today in the BMJ journal RMD Open, Rheumatic and Musculoskeletal Diseases.1 CRYSVITA is licensed in Europe for the treatment of XLH in children and adolescents aged 1 to 17 years with radiographic evidence of bone disease, and in adults.4
▼This medicinal product is subject to additional monitoring.
About X-linked hypophosphataemia
X-linked hypophosphataemia (XLH) is a rare, genetic disease that causes abnormalities in the bones, muscles, and joints.5,6 XLH is not life-threatening, but its burden is life-long and progressive, and it may reduce a person’s quality of life.7
People with XLH have a genetic defect on the X-chromosome, which causes an excessive loss of phosphate through the urine and poor absorption from the gut due to excess of a hormone known as fibroblast growth factor-23 (FGF23), resulting in chronically low levels of phosphate in the blood.4,8 Phosphate is a key mineral needed for maintaining the body’s energy levels, muscle function, and the formation of healthy bones and teeth.9,10 While there is no cure for XLH, therapies aimed at helping to restore and maintain phosphate to normal levels within the body may help to improve the progression of disease symptoms.2
XLH is the most common form of hereditary rickets.11 It can sometimes appear in individuals with no family history of the disease but is usually passed down from a parent who carries the defective gene.12
About CRYSVITA® (burosumab)
CRYSVITA (burosumab) was created and developed by Kyowa Kirin and is a recombinant fully human monoclonal IgG1 antibody against the phosphaturic hormone fibroblast growth factor 23 (FGF23). FGF23 is a hormone that reduces serum levels of phosphate by regulating phosphate excretion and active vitamin D production by the kidney. Phosphate wasting and resulting hypophosphataemia in X-linked hypophosphataemia (XLH) is caused by excess FGF23. CRYSVITA is designed to bind to, and thereby inhibit, the biological activity of FGF23. By blocking excess FGF23 in patients, CRYSVITA is intended to increase phosphate reabsorption from the kidney and increase the production of active vitamin D, which enhances intestinal absorption of phosphate and calcium.
CRYSVITA has been available for clinical use since 2018. The first approval came from the European Commission, that granted a conditional marketing authorisation for CRYSVITA for the treatment of XLH with radiographic evidence of bone disease in children one year of age and older and adolescents with growing skeletons. In 2020, this authorisation was subsequently expanded to include older adolescents and adults.2
CRYSVITA is approved by the US Food and Drug Administration (FDA) for patients with XLH aged 6 months and older and by Health Canada for patients with XLH aged one year and older.13,14
In 2019, CRYSVITA received approval from Japan’s Ministry of Health, Labour and Welfare for the treatment of FGF23-related hypophosphataemic rickets and osteomalacia. In 2020, CRYSVITA was reimbursed by National Health Insurance (NHI) in Japan as a self-injection presentation for the treatment of FGF23-related hypophosphataemic rickets and osteomalacia.
In January 2020, Swissmedic approved CRYSVITA for the treatment of adults, adolescents and children (one year of age and older) with XLH.15
In June 2020, the U.S. Food and Drug Administration (FDA) approved CRYSVITA for patients aged two and older with tumour-induced osteomalacia (TIO), a rare disease that is characterised by the development of tumours that cause weakened and softened bones.16
Kyowa Kirin and Ultragenyx Pharmaceutical Inc. (NASDAQ: RARE: Ultragenyx) have been collaborating in the development and commercialisation of CRYSVITA globally, based on the collaboration and licence agreement between Kyowa Kirin and Ultragenyx.
About Kyowa Kirin
Kyowa Kirin strives to create and deliver novel medicines with life-changing value. As a Japan-based Global Specialty Pharmaceutical Company with a more than 70-year heritage, the company applies cutting-edge science including an expertise in antibody research and engineering, to address the needs of patients and society across multiple therapeutic areas including Nephrology, Oncology, Immunology/Allergy and Neurology. Across our four regions – Japan, Asia Pacific, North America and EMEA/International – we focus on our purpose, to make people smile, and are united by our shared values of commitment to life, teamwork/Wa, innovation, and integrity. You can learn more about the business of Kyowa Kirin at: https://www.kyowakirin.com/
Kyowa Kirin International
http://www.international.kyowa-kirin.com / www.kyowakirin.com
Galabank Business Park
Galashiels, TD1 1QH
United Kingdom
References
1 Briot K, Portale AA, Brandi ML, et al. RMD Open 2021;7:e001714. doi: 10.1136/rmdopen-2021-001714.
2 Insogna KL, Rauch F, Kamenický P, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: a Phase 3, single-arm, international trial. J Bone Miner Res. 2019;34:2183-2191.
3 Portale AA, Carpenter TO, Brandi ML, et al. Calcif Tissue Int 2019;105:271–84.
4 European Medicines Agency. CRYSVITA EPAR product information. Summary of Product Characteristics. Available at: Crysvita, INN-burosumab; (europa.eu). Last updated: June 2021. Last accessed: July 2021.
5 Linglart A, Biosse-Duplan M, Briot K, et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect. 2014;3:R13-30.
6 Haffner D, Emma F, Eastwood DM, et al. Consensus Statement. Evidence-based guideline. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphatemia. Nat Rev Nephrol. 2019;15;435-455.
7 Skrinar A, Dvorak-Ewell M, Evins A, et al. The lifelong impact of X-linked hypophosphatemia: Results from a burden of disease survey. J Endocr Soc. 2019;3:1321-1334.
8 Beck-Nielsen SS, Mughal Z, Haffner D, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14:58.
9 Pesta D, Tsirigotis DN, Befroy DE, et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. The FAESB Journal. 2016;39:3378-3387.
10 Unnanuntana A, Rebolledo BJ, Khair MM, et al. Diseases affecting bone quality: beyond osteoporosis. Clin Orthop Relat Res. 2011;469:2194-2206.
11 Carpenter TO, Imel EA, Holm IA, et al. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26:1381-8.
12 National Center for Advancing Translational Sciences. X-linked hypophosphatemia. Available at: https://rarediseases.info.nih.gov/diseases/12943/x-linked-hypophosphatemia. Last updated: February 2018. Last accessed: July 2021.
13 Health Canada. Regulatory Decision Summary – CRYSVITA. Available at: https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?linkID=RDS00463. Last updated: April 2020. Last accessed: April 2021.
14 Available at : https://www.kyowakirin.com/media_center/news_releases/2019/e20190930_01.html. Last accessed: July 2021
15 Swissmedic. Crysvita, injektionslösung (burosumabum). Available at: https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/new-medicines/vrysvita-injektionsloesung_burosumabum.html. Last updated: January 2020. Last accessed: July 2021.
16 FDA. Available at: FDA Approves First Therapy for Rare Disease that Causes Low Phosphate Blood Levels, Bone Softening | FDA. Last accessed: July 2021
KKI/INT/BUR/1174

Source link
0 notes
Text
Wearing a face-mask can have long term damaging effects

The use of cloth masks may actually be increasing the risk of respiratory illness and viral infections and their global use should be discouraged, according to a UNSW study. (University of New South Wales)
The results of the first randomized clinical trial (RCT) to study the efficacy of cloth masks were published in the journal BMJ Open.
The trial saw 1607 hospital healthcare workers across 14 hospitals in the Vietnamese capital, Hanoi, split into three groups: those wearing medical masks, those wearing cloth masks and a control group based on usual practice, which included mask wearing.
Workers used the mask on every shift for four consecutive weeks.
The study found respiratory infection was much higher among healthcare workers wearing cloth masks.
The penetration of cloth masks by particles was almost 97% compared to medical masks with 44%.
Professor Raina MacIntyre, lead study author and head of UNSW's School of Public Health and Community Medicine, said the results of the study caution against the use of cloth masks.
"Masks are worn to protect from infection during pandemics and outbreaks, especially when there are no drugs or vaccines available for protection," Professor MacIntyre said.
"Masks are especially important for frontline doctors and nurses, as their protection from infection is key to maintaining the ability to tackle a pandemic effectively.
"We should be cautious about cloth mask use in healthcare settings, particularly high-risk situations such as emergency departments, intensive care, paediatric or respiratory wards."

Cloth masks remain widely used globally because they are a cheaper option especially in areas where there are shortages of protective equipment, including in Asian countries, which have historically been affected by emerging infectious diseases, as well as in West Africa, which was the epicentre of a recent Ebola epidemic.
The authors speculate that the cloth masks' moisture retention, their reuse and poor filtration may explain the increased risk of infection.
Professor MacIntyre, who has completed the largest body of clinical trial research on respiratory protection in health workers internationally, said emerging infectious diseases are not constrained within geographical borders.
"Effective controls of outbreaks and pandemics at the origin impacts us directly, so it is important for global disease control that the use of cloth masks be discouraged in high-risk situations," she said.
"Despite more than half the world using cloth masks, global disease control guidelines, including those from the World Health Organisation, fail to clearly specify conditions of their use.
"These guidelines need to be updated to reflect the higher infection risk posed by cloth masks, as found in our study."
Professor MacIntyre said the study's results pointed to the effectiveness of medical masks, in addition to the harm caused by cloth masks.
"Additional research is urgently needed to build on our study's findings."
The trial was a collaboration between researchers in Australia and the National Institute for Hygiene and Epidemiology in Vietnam and was funded by an Australian Research Council Linkage Grant.
A separate expert review by Professor MacIntyre published in tahe British Medical Journal earlier this month found that the lack of research on facemasks and respirators is reflected in varied and sometimes conflicting global policies and guidelines. - Science.Com
Read the full article
0 notes
Text
Managing Surgical Wait Times in the Intra-COVID-19 World
Finding the Right Prioritization Model
By JUSTIN SPECTOR
Restrictions on elective surgical volume in hospitals across the United States are causing a dilemma heretofore unseen in the American healthcare system. Surgeons across services have large and growing backlogs of elective surgeries in an environment where operating room (OR) capacity is restricted due to availability of inpatient beds, personal protective equipment (PPE), staffing, and many other constraints. Fortunately, the U.S. is not the first country to experience and deal with this situation; for many countries, this is the normal state of medicine.
By combining the accumulated experience of health systems around the world with cutting-edge technologies, it is possible to make this crisis manageable for perioperative leadership and, potentially, to improve upon the preexisting models for managing OR time.
The first step in creating an equitable system that can garner widespread buy-in is to agree upon a method for categorizing cases into priority levels. Choosing a system with strong academic backing will help to reduce the influence of intra-hospital politics from derailing the process before it can begin.
Why Cases Should Be Prioritized
If your hospital has a mix of surgeons who perform highly time-sensitive cases — cases where patient quality of life is substantially impacted — as well as cases with minor health or quality of life outcomes, it is important to make sure there will be enough capacity to get the higher urgency cases done within a reasonable amount of time. This allows cases in the backlog to be balanced against new cases that are yet to be scheduled and will help to optimize the flow of patients through the OR.
Case Prioritization Best Practices
The most important feature of a prioritization model is for it to be something your hospital, surgeons and schedulers will be able to understand and be willing to use. For this reason, we have excluded systems that are difficult to implement, such as that used by the Department of Veterans Affairs. The models included, with one exception, have long track records of success, and any hospital should be able to implement them.
Another important consideration in prioritization is what factors you want to consider. These can be broken into three major categories: clinical urgency, risk to the patient, and risk to the hospital. Risk to the patient would include factors such as ASA score, age and any complicating conditions that would make the patient especially at risk if infected with SARS-CoV-2. Clinical urgency — the rate at which the patient’s condition is worsening and the patient’s ability to wait for surgery — is the most straightforward. Finally, risk to the hospital takes into account many factors such as the risk of the patient spreading infection to hospital staff and other patients, their likelihood of needing an ICU bed and blood, and the amount of PPE needed for the case. Each hospital will need to place a different amount of emphasis on each category.
Four Prominent Case Prioritization Models
Descriptive – Surgical Waiting List Info System (SWALIS): SWALIS is a system based on the Italian government’s case prioritization guidelines that solely take into account clinical urgency to assign a case one of five levels, and each priority level is associated with a maximum time before treatment (MTBT). This model is easy to understand and requires minimal administrative oversight, but it does not take into account other factors such as equipment needs and risk to the patient and hospital. Because of the ease of implementation and clarity of the segmentation, this is a favored model.
A model to prioritize access to elective surgery on the basis of clinical urgency and waiting time
Prescriptive – British Columbia Ministry of Health Surgical Patient Registry (SPR): The model used in British Columbia, Canada, is similar to the Italian model in that it divides cases into groups by clinical urgency. However, instead of the surgeon subjectively determining the priority level, this model prescribes a level based on the procedure. This system is especially effective if it will be difficult to instruct your clinics on the prioritization methodology but requires a lot of decisions to be made centrally to determine the priority for each procedure and any modifiers that need to be considered.
Patient Prioritization Codes: Overview
Surgical Wait Time Strategy
Surgical Wait Times – Procedures A-Z
Dental Surgery ‐ Adult – BC List of Patient Condition and Diagnosis Descriptions
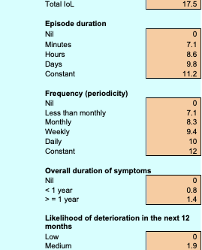
Qualitative Patient Need – General Surgery Prioritization Tool (GSPT): New Zealand was one of the first countries to implement clinical priority assessment criteria (CPAC) nationwide. One CPAC tool being used is the GSPT. It uses a 0-100 scale for each case based on aspects of the impact on the patient’s quality of life and health to determine a relative priority. (See Appendix)
General Surgery Prioritization Tool: a pilot study
Qualitative Multifactor – Medically Necessary, Time-Sensitive (MeNTS): Published in the Journal of the American College of Surgeons, this system uses a mix of subjective and objective scores in different categories to create a cumulative score between 21 and 105. Higher scores equate to a greater risk to reward for the procedure. The process of scoring each case requires 21 factors to be rated on a scale of 1 to 5, making this best suited to systems with strong admin staff who can complete these evaluations for each case.
Medically Necessary, Time-Sensitive Procedures: Scoring System to Ethically and Efficiently Manage Resource Scarcity and Provider Risk During the COVID-19 Pandemic
Choosing the Right Model
The two most important factors when designing a prioritization model for your hospital or system are consistency and compliance. It is important that across surgeons and service lines there is an understanding of what priority level is appropriate and that those are in line with leadership’s intent. It is also important to consider the ease of use as models that require too much work per individual case may be ignored or half-heartedly complied with.
As there has been little national or state-level guidance on this subject, each hospital organization has a great deal of leeway to choose a method that fits their unique needs.
Other Considerations
If your hospital is constrained on beds, PPE, blood or any other surgical input, consider adding these as factors in your prioritization system. For example, if PPE is a constraint, it’s important to balance case lengths as shorter cases result in more PPE use throughout a surgery day. Similarly, if inpatient beds are limited, you may want to set a threshold on the total number of cases that require greater than six hours of in-bed recovery time. This is an area where web-based tools excel due to their ability to leverage sophisticated packing algorithms.
Translating Priority Scores Into Surgery Dates
In the American healthcare system, surgical schedules are built around block allocation. This means that each case’s score is not enough to determine when it should be performed since the surgeons will not be coming in to do just one case. Each surgeon or department’s entire backlog should be considered to determine how surgical time should be distributed.
The challenge is to balance priority against wait time and overall backlog volume. While certain service lines may only have low- to medium-priority cases, if their caseload is sufficient, then they should be given time even within the first few weeks of opening up elective booking. Using a scoring system for each case that takes into account wait time, along with determining the MTBT for each priority category, it is possible to formulate how much time each surgeon or service should receive each week. Because surgeons’ waitlists are constantly changing, it is useful to repeat this exercise at a weekly or biweekly cadence to ensure optimal distribution.
Further Reading
Below are academic papers that discuss the importance and process of prioritization that may also be useful. The author would like to thank Dr. James Caldwell, director of surgical services, Parkview Medical Center, for his assistance in compiling this information.
Edwards RT. Points for pain: waiting list priority scoring systems. BMJ. 1999;318(7181):412–414; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1114887/
Curtis AJ, Russell COH, Stoelwinder JU, McNeil JJ (2010). Waiting Lists and Elective Surgery: Ordering the Queue. Medical Journal of Australia. 192: 217-220. doi:10.5694/j.1326-5377.2010.tb03482.x; https://www.ncbi.nlm.nih.gov/pubmed/20170460
COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures (Online March 17, 2020); https://www.facs.org/covid-19/clinical-guidance/triage
Mullen P M (2003). Prioritising waiting lists: how and why? European Journal of Operational Research, 150(1), 32–45. doi: 10.1016/S0377-2217(02)00779-8; https://www.sciencedirect.com/science/article/abs/pii/S0377221702007798?via%3Dihub
Davis B, Johnson SR. Real-time priority scoring system must be used for prioritisation on waiting lists. BMJ. 1999;318(7199):1699. doi:10.1136/bmj.318.7199.1699; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1116039/
Testi A, Tanfani E, Valente R, Ansaldo GL, Torre GC. Prioritizing Surgical Waiting Lists. Journal of Evaluation in Clinical Practice. 2006; ISSN 1356-1294; https://pubmed.ncbi.nlm.nih.gov/18211645/
Appendix
Scoring system for NZ, GSPT
Justin Spector is a product manager at LeanTaaS where he is continuously enhancing ways to help hospitals ease access to OR time and optimize OR utilization.
Managing Surgical Wait Times in the Intra-COVID-19 World published first on https://venabeahan.tumblr.com
0 notes
Text
Managing Surgical Wait Times in the Intra-COVID-19 World
Finding the Right Prioritization Model
By JUSTIN SPECTOR
Restrictions on elective surgical volume in hospitals across the United States are causing a dilemma heretofore unseen in the American healthcare system. Surgeons across services have large and growing backlogs of elective surgeries in an environment where operating room (OR) capacity is restricted due to availability of inpatient beds, personal protective equipment (PPE), staffing, and many other constraints. Fortunately, the U.S. is not the first country to experience and deal with this situation; for many countries, this is the normal state of medicine.
By combining the accumulated experience of health systems around the world with cutting-edge technologies, it is possible to make this crisis manageable for perioperative leadership and, potentially, to improve upon the preexisting models for managing OR time.
The first step in creating an equitable system that can garner widespread buy-in is to agree upon a method for categorizing cases into priority levels. Choosing a system with strong academic backing will help to reduce the influence of intra-hospital politics from derailing the process before it can begin.
Why Cases Should Be Prioritized
If your hospital has a mix of surgeons who perform highly time-sensitive cases — cases where patient quality of life is substantially impacted — as well as cases with minor health or quality of life outcomes, it is important to make sure there will be enough capacity to get the higher urgency cases done within a reasonable amount of time. This allows cases in the backlog to be balanced against new cases that are yet to be scheduled and will help to optimize the flow of patients through the OR.
Case Prioritization Best Practices
The most important feature of a prioritization model is for it to be something your hospital, surgeons and schedulers will be able to understand and be willing to use. For this reason, we have excluded systems that are difficult to implement, such as that used by the Department of Veterans Affairs. The models included, with one exception, have long track records of success, and any hospital should be able to implement them.
Another important consideration in prioritization is what factors you want to consider. These can be broken into three major categories: clinical urgency, risk to the patient, and risk to the hospital. Risk to the patient would include factors such as ASA score, age and any complicating conditions that would make the patient especially at risk if infected with SARS-CoV-2. Clinical urgency — the rate at which the patient’s condition is worsening and the patient’s ability to wait for surgery — is the most straightforward. Finally, risk to the hospital takes into account many factors such as the risk of the patient spreading infection to hospital staff and other patients, their likelihood of needing an ICU bed and blood, and the amount of PPE needed for the case. Each hospital will need to place a different amount of emphasis on each category.
Four Prominent Case Prioritization Models
Descriptive – Surgical Waiting List Info System (SWALIS): SWALIS is a system based on the Italian government’s case prioritization guidelines that solely take into account clinical urgency to assign a case one of five levels, and each priority level is associated with a maximum time before treatment (MTBT). This model is easy to understand and requires minimal administrative oversight, but it does not take into account other factors such as equipment needs and risk to the patient and hospital. Because of the ease of implementation and clarity of the segmentation, this is a favored model.
A model to prioritize access to elective surgery on the basis of clinical urgency and waiting time
Prescriptive – British Columbia Ministry of Health Surgical Patient Registry (SPR): The model used in British Columbia, Canada, is similar to the Italian model in that it divides cases into groups by clinical urgency. However, instead of the surgeon subjectively determining the priority level, this model prescribes a level based on the procedure. This system is especially effective if it will be difficult to instruct your clinics on the prioritization methodology but requires a lot of decisions to be made centrally to determine the priority for each procedure and any modifiers that need to be considered.
Patient Prioritization Codes: Overview
Surgical Wait Time Strategy
Surgical Wait Times – Procedures A-Z
Dental Surgery ‐ Adult – BC List of Patient Condition and Diagnosis Descriptions
Qualitative Patient Need – General Surgery Prioritization Tool (GSPT): New Zealand was one of the first countries to implement clinical priority assessment criteria (CPAC) nationwide. One CPAC tool being used is the GSPT. It uses a 0-100 scale for each case based on aspects of the impact on the patient’s quality of life and health to determine a relative priority. (See Appendix)
General Surgery Prioritization Tool: a pilot study
Qualitative Multifactor – Medically Necessary, Time-Sensitive (MeNTS): Published in the Journal of the American College of Surgeons, this system uses a mix of subjective and objective scores in different categories to create a cumulative score between 21 and 105. Higher scores equate to a greater risk to reward for the procedure. The process of scoring each case requires 21 factors to be rated on a scale of 1 to 5, making this best suited to systems with strong admin staff who can complete these evaluations for each case.
Medically Necessary, Time-Sensitive Procedures: Scoring System to Ethically and Efficiently Manage Resource Scarcity and Provider Risk During the COVID-19 Pandemic
Choosing the Right Model
The two most important factors when designing a prioritization model for your hospital or system are consistency and compliance. It is important that across surgeons and service lines there is an understanding of what priority level is appropriate and that those are in line with leadership’s intent. It is also important to consider the ease of use as models that require too much work per individual case may be ignored or half-heartedly complied with.
As there has been little national or state-level guidance on this subject, each hospital organization has a great deal of leeway to choose a method that fits their unique needs.
Other Considerations
If your hospital is constrained on beds, PPE, blood or any other surgical input, consider adding these as factors in your prioritization system. For example, if PPE is a constraint, it’s important to balance case lengths as shorter cases result in more PPE use throughout a surgery day. Similarly, if inpatient beds are limited, you may want to set a threshold on the total number of cases that require greater than six hours of in-bed recovery time. This is an area where web-based tools excel due to their ability to leverage sophisticated packing algorithms.
Translating Priority Scores Into Surgery Dates
In the American healthcare system, surgical schedules are built around block allocation. This means that each case’s score is not enough to determine when it should be performed since the surgeons will not be coming in to do just one case. Each surgeon or department’s entire backlog should be considered to determine how surgical time should be distributed.
The challenge is to balance priority against wait time and overall backlog volume. While certain service lines may only have low- to medium-priority cases, if their caseload is sufficient, then they should be given time even within the first few weeks of opening up elective booking. Using a scoring system for each case that takes into account wait time, along with determining the MTBT for each priority category, it is possible to formulate how much time each surgeon or service should receive each week. Because surgeons’ waitlists are constantly changing, it is useful to repeat this exercise at a weekly or biweekly cadence to ensure optimal distribution.
Further Reading
Below are academic papers that discuss the importance and process of prioritization that may also be useful. The author would like to thank Dr. James Caldwell, director of surgical services, Parkview Medical Center, for his assistance in compiling this information.
Edwards RT. Points for pain: waiting list priority scoring systems. BMJ. 1999;318(7181):412–414; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1114887/
Curtis AJ, Russell COH, Stoelwinder JU, McNeil JJ (2010). Waiting Lists and Elective Surgery: Ordering the Queue. Medical Journal of Australia. 192: 217-220. doi:10.5694/j.1326-5377.2010.tb03482.x; https://www.ncbi.nlm.nih.gov/pubmed/20170460
COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures (Online March 17, 2020); https://www.facs.org/covid-19/clinical-guidance/triage
Mullen P M (2003). Prioritising waiting lists: how and why? European Journal of Operational Research, 150(1), 32–45. doi: 10.1016/S0377-2217(02)00779-8; https://www.sciencedirect.com/science/article/abs/pii/S0377221702007798?via%3Dihub
Davis B, Johnson SR. Real-time priority scoring system must be used for prioritisation on waiting lists. BMJ. 1999;318(7199):1699. doi:10.1136/bmj.318.7199.1699; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1116039/
Testi A, Tanfani E, Valente R, Ansaldo GL, Torre GC. Prioritizing Surgical Waiting Lists. Journal of Evaluation in Clinical Practice. 2006; ISSN 1356-1294; https://pubmed.ncbi.nlm.nih.gov/18211645/
Appendix
Scoring system for NZ, GSPT

Justin Spector is a product manager at LeanTaaS where he is continuously enhancing ways to help hospitals ease access to OR time and optimize OR utilization.
Managing Surgical Wait Times in the Intra-COVID-19 World published first on https://wittooth.tumblr.com/
0 notes
Text
Mobile phones potential carrier of coronavirus, hospitals should restrict their use: Doctors - Times of India
New Post has been published on https://apzweb.com/mobile-phones-potential-carrier-of-coronavirus-hospitals-should-restrict-their-use-doctors-times-of-india/
Mobile phones potential carrier of coronavirus, hospitals should restrict their use: Doctors - Times of India

NEW DELHI: A group of doctors from the AIIMS, Raipur has recommended restrictions on use of mobile phones in healthcare institutions amid the COVID-19 pandemic, warning that such devices can be a potential carrier of the virus and lead to infection among healthcare workers.
In a commentary published in the BMJ Global Health journal, the doctors stated that mobile phone surfaces are a peculiar ‘high-risk’ surface, which can directly come in contact with the face or mouth, even if hands are properly washed and one study indicates that some healthcare workers use phones every 15 minutes to two hours.
Though there have been many significant guidelines from various health organisations like the WHO and CDC focusing on prevention and control of disease, the commentary highlighted “there is no mention of or focus on mobile phones in these guidelines, including the WHO infection control and prevention guidelines, which recommends the use of hand washing”.
In healthcare facilities, phones are used to communicate with other health care workers, look up recent medical guidelines, research drug interactions, understand adverse events and side effects, conduct telemedicine appointments and track patients among others, stated the document.
The document has been authored by Dr Vineet Kumar Pathak, Dr Sunil Kumar Panigrahi, Dr M Mohan Kumar, Dr Utsav Raj and Dr Karpaga Priya P from the Department of Community and Family Medicine.
“In their tendency to come in direct contact with the face, nose or eyes in healthcare settings, mobile phones are perhaps second only to masks, caps or goggles,” the authors said.
“However, they are neither disposable nor washable like these other three, thus warranting disinfection. Mobile phones can effectively negate hand hygiene… There is growing evidence that mobile phones are a potential vector for pathogenic organisms,” they said.
It is the need of the hour to address proper hygienic use of mobile phones in healthcare settings. In a study in India, almost 100 per cent of health workers of a tertiary care hospital used mobile phones in the hospital, but only 10 per cent of them had at any time wiped their mobile phones clean, the commentary published on April 22 said.
“The safest thing to do is to consider your phone as an extension of your hand, so remember you are transferring whatever is on your phone to your hand,” Dr Pathak said.
Amidst the ongoing pandemic, two biggest mobile phone companies have uploaded their user support guidelines, saying that 70 pc isopropyl alcohol or Clorox Disinfecting Wipes can be used to gently wipe the exterior surface of phones in switched-off mode.
However, in doing so, the use of bleach or entry of moisture through any of the openings must be avoided, and any harsh chemical may damage the oleophobic screen, leading to damage in the touch screen sensitivity of the phone, the article stated.
Mobile phones are one of the most highly touched surfaces according to the Centers for Disease Control and Prevention (CDC), along with counters, tabletops, doorknobs, bathroom fixtures, toilets, keyboards, tablets and bedside tables.
The doctors recommended restriction on mobile phone usage in healthcare settings like hospital wards, ICUs and operation theatres, while advocating use of headphones to prevent contact with the face while talking.
There should be no sharing of mobile phones, headphones or headsets of any kind. In addition, where available, the use of interdepartmental intercom facility may be promoted.
“Although hand hygiene and mobile phone use by a person are not mutually exclusive, it is high time to acknowledge the potential role of mobile phones in disease transmission cascade and to take evidence-based appropriate actions. This is especially important, given the ongoing COVID-19 pandemic,” the authors said.
They said it is necessary for government agencies and the WHO to generate public awareness and to formulate suitable information, education and communication material on mobile phone hygiene, especially in healthcare settings.
AIIMS, New Delhi, Resident Doctors’ Association (RDA) General Secretary, Dr Srinivas Rajkumar T said even outside health care settings, people should pay special attention to usage of mobile phones as they carry them to all places.
“Phone and computer peripherals like keyboard, mouse, etc. should be covered with transparent plastic covers which can be cleaned without interfering with their function. Cleaning hands by soap or alcohol based hand sanitizer before and after contact with phone and between contact with other surfaces can decrease the risk of potential transmission.
“Using handsfree headset, dedicated operator/assistant per ward handling the communication via common line in hospitals while on duty can enable communication without compromising safety,” Dr Srinivas said
Source link
0 notes
Photo

Sitting for more than three hours a day can cut two years off a person’s life expectancy, even if he or she exercises regularly, a new study finds. Watching TV for more than two hours a day can shorten life expectancy even further, by another 1.4 years. The findings suggest that when it comes to gleaning health benefits from physical activity, it may not be enough just to get the recommended amount of daily exercise — the government advises about a half-hour of moderate activity a day for adults. But what about the other 23.5 hours of every day? Researchers say it’s important not to spend it sedentary or sitting. In the same way that both pushing the gas and hitting the brake can adjust the speed of your car, researchers say that physical activity and sedentary behavior independently affect your health and life expectancy. Whether "you're physically active and meet the exercise guidelines, or if you’re not active," says Peter Katzmarzyk, professor of epidemiology at Pennington Biomedical Research Center and lead author of the new paper published in the online journal BMJ Open, "sitting is bad." #followme #exercise #health #folow #instagood #sadistar https://www.instagram.com/p/B4AldJnjYwQ/?igshid=bsmwkjmzy0ek
0 notes
Text
Commercial interests, transparency, and independence: a call for submissions
PDF
Commercial interests, transparency, and independence: a call for submissions. Help the move towards independence from commercial interests.
Submissions are welcome from now, with a final deadline of 15 January 2020.
Ray Moynihan assistant professor 1, Helen Macdonald UK research editor 2, Carl Heneghan professor,
Lisa Bero professor 4, Fiona Godlee editor in chief 2
1Centre for Research in Evidence-Based Practice, Bond University, Gold Coast, Australia; 2The BMJ, London, UK; 3Nuffield Department of Primary
Care, Oxford University, Oxford, UK; 4Charles Perkins Centre, University of Sydney, Sydney, Australia
A decade ago the US Institute of Medicine (IOM) issued a landmark report on conflicts of interest in research, medical education, and practice.1 Highlighting benefits of collaborations between physicians, researchers, and companies to develop new products that can improve health, the report also raised substantial concerns that extensive financial ties could unduly influence professional judgments. It concluded these financial conflicts of interest could jeopardise the integrity of science, the objectivity of education, the quality of care, and public trust in medicine. The report recommended more research on conflicts of interest, improvements in transparency, and greater independence from industry.
Today we announce plans for a stream of BMJ content to revisit these concerns and ask you to join us. A key aim is to identify and respond to commercial influences on health and healthcare, to understand under what circumstances involvement with industry is truly necessary. Where it is not necessary, we want to forge a new independence from those who make and sell products, to strengthen trust in how evidence is produced and disseminated, and to drive more rational and safer use of drugs, devices, diagnoses, and data in the public interest.
Problematic relationships
Since the 2009 IOM report, transparency has improved, but key recommended steps towards independence—such as prohibiting free meals, excluding conflicted authors from guidelines, and ending industry influenced medical education—have not been taken. These practices are still widespread despite continuing evidence of distorting impacts on research and practice. A 2010 cross sectional review found that the views of “key opinion leaders” strongly correlate with their sponsor’s interests.2 A 2016 study of 279 000 physicians, using the new US Open Payments transparency initiative (https://www.cms.gov/ openpayments/), found an association between receipt of just one promotional meal and higher prescriptions of the sponsors’ drugs.3 A 2017 Cochrane review has confirmed that sponsored clinical trials tend to find more favourable outcomes about sponsors’ products.4 In 2018 new evidence has identified ongoing sponsor involvement in design conduct and reporting of research, and a lack of transparency around such
involvement.5
Other work shows how companies can control information about their products by selectively publishing or suppressing data and even by changing the standards used to evaluate research,6 as described in figure 1. Investigative journalism continues to expose cases where financial interests have contributed to patient harm, as occurred with diabetes drug rosiglitazone,7 with infant formula,8 and with vaginal mesh.9 These examples bear witness to inadequate regulation, aggressive marketing, and a research
establishment and medical profession still firmly entangled with industry.
Graphic:
Industry strategies to influence evidence and discourse about evidence
The past decade has seen a growing and related understanding of the threat to human health from overdiagnosis and too much medicine (https://www.bmj.com/too-much-medicine). Recent research confirms the extent to which this medical excess is driven by commercial influences, including on disease definitions.10 By failing to mitigate these influences we overmedicalise society by labelling healthy people as sick, causing unnecessary cost to health systems and harm to patients.
The BMJ’s response so far
The 2009 IOM report described transparency as a “critical but limited first step in the process of identifying and responding to conflicts of interest.” It suggested that if medical organisations did not act to reduce conflicts then pressure would likely mount for external regulation. Taking up this challenge, The BMJ has updated its policies around ties with commercial companies
(box 1).
Box 1: Efforts to increase transparency and independence from. commercial interests at The BMJ
2014: We stopped publishing educational content or clinical editorials from authors who have relevant financial ties to commercial organisations11
2016: We introduced a series of clinical guidelines called BMJ Rapid Recommendations. Here the same policies of independence apply to a large panel, and include additional tougher policies for managing intellectual interests, and involving patients and the public12
2017: We began annual disclosure of all income from industry advertising and sponsorship13
2019: We stopped accepting advertisments for breast milk substitutes following our investigation into its overpromotion for children who do not need them8
These changes show that The BMJ aims not only to describe the, problem but to be part of the solution. They have led to difficult conversations, harder work, increased costs, or loss of revenue. Other organisations will have considered or taken similar steps. We would like to hear about these discussions and reforms, and where possible, outcomes and impact. We also welcome your thoughts on what we should focus on next. The BMJ has also worked with the Centre for Evidence Based Medicine at Oxford University and a global community of evidence based medicine supporters to frame a manifesto for better evidence in medicine.14 One of the manifesto’s nine commitments is to reduce conflicts of interest to facilitate better creation, translation, and use of evidence. Our jointly hosted conference, EBM Live, will include a themed day on conflicts of interest in July 2019. We recognise that taking a strong stance on commercial interests is just one piece of a wider picture of other financial and also non-financial interests. There are many differing perspectives, including from those who question assumptions about the dangers of conflicts of interest.15 By focusing on commercial financial interests we do not aim to minimise or avoid this wider discussion.
Call for submissions
Today, The BMJ launches a call for submissions for a themed collection (box 2). New transparency initiatives such as US Open Payments provide invaluable data, and we welcome
research drawing on these new sources. There are also new and important relationships to study: those with commercial entities beyond drug companies, and those between industry and patient and consumer advocacy groups, which are rightly increasingly influential in the creation and use of evidence.
Box 2: Call for submissions to The BMJ themed collection on commercial interests, transparency, independence
Aims of the collection
• To better understand the nature of commercial conflicts of interest
• To examine how commercial interests effect health and healthcare, including health research, practice, and education
• To explore when commercial ties are truly necessary and when independence is most needed
• To share examples of progress from transparency to independence
What are we looking for?
• Submissions for the themed collection are open across all article formats for The BMJ. We seek original data, qualitative and quantitative analysis, as well as evaluated examples of groups or organisations forging genuine independence from industry
• We are particularly interested in submissions about industry’s involvement in evidence creation, evaluation, synthesis and translation into guidelines, and about moves towards independence in these processes
• We welcome submissions about the interests of commercial organisations producing any products that effect health, including drugs, devices, food, drink, insurance, social media, and information technology
• We welcome exploration of ties between industry and all groups relevant to health, including healthcare professional, researchers, and patient or consumer advocacy groups
We may offer publication of selected research articles in our sister journal BMJ Open rather than The BMJ, with an online link to the collection.
Submissions are welcome from now, with a final deadline of 15 January 2020.
Normal publication processes for each journal will apply. For feedback on ideas before submission please contact
[email protected]
We intend to launch initial material at the Preventing Overdiagnosis conference in Sydney on 5-7 December 2019
(www.preventingoverdiagnosis.net) and the full collection at EBM Live in July 2020 (ebmlive.org). We look forward to
receiving your work.
We thank Meng Koach for the figure.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: RM is funded by a National Health
and Medical Research Council fellowship, GNT1124207, and hosts a podcast funded by Cochrane Australia. The idea for this collection of content was suggested
by RM and adapted in collaboration with the listed authors. FG and HM are editors of The BMJ, which is committed to increasing transparency and ensuring that evidence and practice are independent from financial interests. The BMJ receives income from commercial advertisers and sponsors, declared annually at https://
www.bmj.com/about-bmj/publishing-model. RM has a longstanding interest in conflicts of interest, enhanced transparency, and more independence from
commercial interests in healthcare and is cochair of the Preventing Overdiagnosis scientific committee. CH has received expenses and fees for his media work, and
he holds grant funding from the NIHR, the NIHR School of Primary Care Research and The NIHR Oxford BRC. CH is Editor in Chief of BMJ Evidence-Based Medicine, an NHS urgent care GP and Director of CEBM, which jointly runs the EvidenceLive Conference with the BMJ and the Overdiagnosis Conference with
international partners based on a non-profit model. RM and LB are guest editors of the BMJ themed collection on commercial interests.
Provenance and peer review: Commissioned; not externally peer reviewed.
1 Lo B, Field MJ. Conflict of interest in medical research, education, and practice. National
Academies Press, 2009.
2 Wang AT, McCoy CP, Murad MH, Montori VM. Association between industry affiliation
and position on cardiovascular risk with rosiglitazone: cross sectional systematic review.
BMJ 2010;340:c1344. 10.1136/bmj.c1344 20299696
3 DeJong C, Aguilar T, Tseng CW, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical
industry-sponsored meals and physician prescribing patterns for Medicare beneficiaries.
JAMA Intern Med 2016;176:1114-22. 10.1001/jamainternmed.2016.2765 27322350
4 Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research
outcome. Cochrane Database Syst Rev 2017;2:MR000033.28207928
5 Rochon PA, Stall NM, Savage RD, Chan AW. Transparency in clinical trial reporting. BMJ
2018;363:k4224. 10.1136/bmj.k4224 30301724
6 Bero L. Ten tips for spotting industry involvement in science policy. Tob Control
2018;28:1-2.29941543
For personal use only: See rights and reprints http://www.bmj.com/permissions Subscribe: http://www.bmj.com/subscribe
BMJ 2019;365:l1706 doi: 10.1136/bmj.l1706 (Published 16 April 2019) Page 2 of 3
EDITORIALS
BMJ: first published as 10.1136/bmj.l1706 on 16 April 2019. Downloaded from http://www.bmj.com/ on 19 April 2019 by guest. Protected by copyright.
7 Cohen D. Rosiglitazone: what went wrong?BMJ 2010;341:c4848.
10.1136/bmj.c4848 20819889
8 Godlee F. Disentangling ourselves from “big formula.”BMJ
2018;363:k514610.1136/bmj.k5146 .
9 Heneghan C, Aronson JK, Goldacre B, Mahtani KR, Plüddemann A, Onakpoya I.
Transvaginal mesh failure: lessons for regulation of implantable devices. BMJ
2017;359:j5515. 10.1136/bmj.j5515 29217786
10 Pathirana T, Clark J, Moynihan R. Mapping the drivers of overdiagnosis to potential
solutions. BMJ 2017;358:j3879. 10.1136/bmj.j3879 28814436
11 Chew M, Brizzell C, Abbasi K, Godlee F. Medical journals and industry ties. BMJ
2014;349:g7197. 10.1136/bmj.g7197 25432164
12 Siemieniuk RA, Agoritsas T, Macdonald H, Guyatt GH, Brandt L, Vandvik PO. Introduction
to BMJ Rapid Recommendations. BMJ 2016;354:i5191. 10.1136/bmj.i5191 27680768
13 Godlee F, Abbasi K, Bloom T. BMJ declares its revenues from industry. BMJ
2017;359:j4930. 10.1136/bmj.j4930 29070599
14 Heneghan C, Mahtani KR, Goldacre B, Godlee F, Macdonald H, Jarvies D. Evidence
based medicine manifesto for better healthcare. BMJ 2017;357:j2973.
10.1136/bmj.j2973 28634227
15 Rosenbaum L. Conflicts of interest: part 1: reconnecting the dots–reinterpreting
industry-physician relations. N Engl J Med 2015;372:1860-4.
10.1056/NEJMms1502493 25946288
Published by the BMJ Publishing Group Limited. For permission to use (where not already
granted under a licence) please go to http://group.bmj.com/group/rights-licensing/
permissions
For personal use only: See rights and reprints http://www.bmj.com/permissions Subscribe: http://www.bmj.com/subscribe
BMJ 2019;365:l1706 doi: 10.1136/bmj.l1706 (Published 16 April 2019) Page 3 of 3
EDITORIALS
BMJ: first published as 10.1136/bmj.l1706 on 16 April 2019. Downloaded from http://www.bmj.com/ on 19 April 2019 by guest. Protected by copyright.
Commercial interests, transparency, and independence: a call for submissions was originally published on Baby Milk Action
0 notes
Text
Dietary supplements Could Decrease Ldl cholesterol — However They Can Injury Your Liver

Many individuals use dietary dietary supplements on prime of or rather than prescription medicines to handle their well being circumstances.
However with out strict federal oversight or complete research, these dietary supplements can typically trigger critical medical points.
For one 64-year-old girl who took a crimson yeast rice complement to decrease her ldl cholesterol, the top end result was a visit to the hospital for acute liver harm.
A gaggle of docs from Michigan described the lady's case within the journal BMJ Case Stories. They famous that she took the crimson yeast rice complement as a result of she was "hesitant to start taking statins."
Crimson yeast rice dietary supplements comprise monacolin Okay, starting from . This compound is chemically equivalent to the lively ingredient within the cholesterol-lowering drug lovastatin, which additionally carries a threat of liver harm.
After utilizing the complement for six weeks, the lady ended up within the hospital with signs together with fatigue, bloating, and darkish urine.
Her docs identified her with drug-induced liver harm, with crimson yeast rice because the possible trigger. She was handled with steroids till her situation improved.
Though this can be a single affected person, it raises questions in regards to the security of crimson yeast rice dietary supplements and the way effectively they're regulated in the USA.
Is crimson yeast rice protected and efficient?
Some research have discovered that crimson yeast rice can decrease LDL ("bad") ldl cholesterol -- both by itself or with different pure compounds.
Dr. Jay Mohan, a heart specialist at McLaren Macomb Hospital and McLaren Oakland Medical Middle within the Detroit space stated "it's very interesting to know that there are other options to statins. But the problem is that the safety profile of red yeast rice is unpredictable."
Different case experiences have recognized critical negative effects in individuals who used crimson yeast rice.
Nevertheless, one evaluate of earlier analysis discovered that most individuals tolerated crimson yeast rice.
The authors level out that scientific research use high-quality crimson yeast rice. The problem is what you purchase on-line or within the retailer could not dwell as much as these exact requirements.
In the USA, dietary supplements do not need to bear the identical scientific testing as pharmaceuticals to indicate that they're protected and efficient.
And firms that make dietary supplements do not need to observe the identical strict manufacturing guidelines as pharmaceutical corporations.
This may increasingly change sooner or later. The U.S. Meals and Drug Administration lately introduced that it'll strengthen its oversight of the dietary complement trade.
However proper now, this lack of oversight implies that a crimson yeast rice complement from one firm could have a lot greater quantities of the lively ingredient monacolin Okay. It might additionally produce other compounds that trigger negative effects or work together with prescription medicines.
"You don't know how much of an ingredient you have in each pill," stated Dr. Colin Zhu, a touring doctor who focuses on household observe, life-style drugs, and culinary drugs.
"On top of that," stated Zhu, "you don't know where the company gets the ingredients from, or what kind of quality control they have, in terms of processing and manufacturing."
The BMJ Case Stories article brings house the purpose that "natural" would not all the time imply "safe."
"The fact that the patient developed drug-induced liver injury from taking a supplement like this is very concerning," stated Mohan, "because that could be a life-threatening illness."
Speaking to your physician about complement use
Mohan stated it will be troublesome for a lot of cardiologists to advocate a crimson yeast rice complement over statins, as a result of statins are an necessary a part of how they deal with sufferers with excessive ldl cholesterol.
Statins even have years of scientific trials behind them exhibiting their advantages. The analysis behind crimson yeast rice is much less intensive.
In fact, statins have their very own negative effects, together with muscle-related points, new-onset diabetes, and an elevated threat of stroke.
However 85 to 90 % of individuals report no negative effects from these medicine, in accordance with the American Faculty of Cardiology.
"The statin medications we have today are so well-tolerated, and they've been so extensively tested, that I rarely have patients that need to be taken off these medications," stated Mohan.
Medical doctors additionally observe up with sufferers after they begin taking a statin to see how effectively they're tolerating it. This contains checking their blood ranges for issues associated to the drug.
This ongoing monitoring piece is lacking when folks self-treat utilizing dietary dietary supplements, particularly after they do not speak with their physician about what they're taking.
Zhu stated he would not advocate that sufferers self-treat with dietary supplements, though he admits that many individuals already do.
One latest estimates that over half of Individuals took a minimum of one dietary complement over the previous 30 days.
Additionally, in accordance with Client Stories, Individuals spent about $49 million on crimson yeast rice dietary supplements in 2015.
Mohan stated he encourages sufferers to let him know if they do not wish to take a drugs he has prescribed. And to speak to him about dietary supplements they're taking.
As for crimson yeast rice complement? He can be open to a affected person making an attempt it.
"But I would let them know the risks," stated Mohan. "And I would also tell them that this supplement isn't shown to have the same beneficial effects as statins, which it is meant to replace."
Zhu agrees that it is vital for folks to speak with their major care supplier about what dietary supplements they're taking.
"But it's not just about supplementing. It's about finding the root cause of disease," stated Zhu. "If there's a reason why you're supplementing, that needs to be clarified with your primary care provider."
Read the full article
0 notes