#fda reporting
Link
Chapters: 3/?
Fandom: Star Wars: The Clone Wars (2008) - All Media Types
Rating: Not Rated
Warnings: Creator Chose Not To Use Archive Warnings
Relationships: Clone Troopers & Jedi Order (Star Wars)
Characters: Original Clone Trooper Character(s) (Star Wars), Jedi
Additional Tags: Paperwork, Drabble Collection, Names, Clone Troopers Deserve Better (Star Wars), Kaminoans Being Assholes (Star Wars), Kaminoan Eugenics (Star Wars), Clone Trooper Dehumanization (Star Wars)
Summary:
A look at the daily life during the clone wars on Kamino and in the GAR through the perspective of paperwork
Each chapter has its own rating and warning in the notes.
Chapter 3: Inspection report of Kaminoan facilities by the Jedi order
Warnings: Clone Trooper decommissioning
#Tcw#clone wars#decommissioning#Clone Trooper decommissioning#drabble#it's based on an FDA 483 report#and if that doesn't scream riveting reading material I don't know what does#petrifiedforests writes#fanfiction#Kaminoa
8 notes
·
View notes
Text
i fucking hate taking vitamin tablets right i think thats nasty as hell so instead i have vitamin gummies and those dissolvable lads u slap in your water and get insta yummy fizzie vitamin drink from :-) being an adult means im healthy and i take my vitamins in a FUN WAY
#getting these gummies sent me into a hugeass research spiral about american vitamins#the labels on vitamins do not have to be accurate. the fda does not review what is in vitamins.#one of the gummies i bought is an american brand and i went deep into research spirals includong finding lab analyses#of one of the gummies and an accuracy report on the ingredients#this brand is ok but god. i will never take a vitamin in the us that scared the shit outta me#theres heavy metals in some o those vitamins. yikers
12 notes
·
View notes
Text
Didn't know that medical appointments would turn into a fun game of "Will I receive actual medical treatment or have a nurse threaten to have me arrested again?"
#i have an appointment in 2 days and i am SO nervous#i keep thinking about how that damn nurse fucked up my life#how are you going to claim someone is a DUI suspect when they don't even drive ??? HELLO ????? like are you fucking kidding me ??????????#and say they are taking more meds than what they're actually taking#and send a report full of these bullshit lies to the psychiatric ward of the hospital#because the nurse says that she finds you ~concerning~#and then she recommends non-FDA-approved herbs and intermittent fasting as 'medical treatments'#'cause fuck science right?#like obviouslyyyyy the reason i'm mentally ill is because i don't take enough herbal supplements or fast 🤪 /sarcasm#the mean girl to nurse pipeline is so real#i really wish i could get her fired#fuck you nurse b*****a
3 notes
·
View notes
Text
Il secolo di prove che i vaccini causano morti infantili
C’è un secolo di prove che collegano i vaccini alla morte infantile improvvisa e numerosi attivisti hanno cercato di costringere il governo federale a indagare per decenni
Source: 24 ago 2022; by A Midwestern Doctor on The Forgotten Side of Medicine
Continue reading Untitled

View On WordPress
#Autopsia#Bambini#Batteri#Case Report#Casi SIDS#Causa Morte#Causalità#Cecità Medica#Corruzione Politica#Dogma#Effetti Collaterali#Encefalopatia#Etica#Eventi Avversi#Farmaci#Farmaci Sperimentali#FDA#Gemelli#Giuramento Ippocrate#Governo Federale#Indagini#Indagini Giudiziarie#Industria Farmaceutica#Infezione#Medicare#Medicina Allopatica#Mercati Garantiti#Mercati Vincolati#Monitoraggio#Morte in Culla
4 notes
·
View notes
Text
going fucking insane bc i have to choose a project based on the results from the weighted scoring model

which by this, project 3 is the objective best.
HOWEVER.
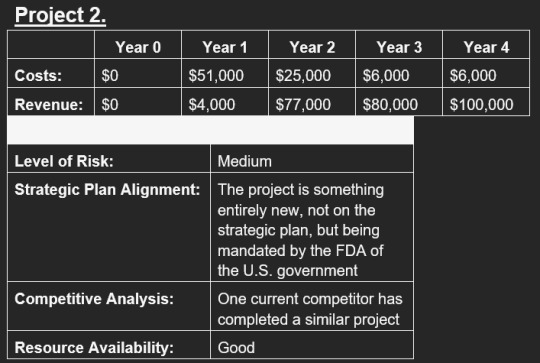
in the description for project 2, it says it’s mandated. mandated. if it’s fucking MANDATED then why the fuck am i doing this analysis in the first place??????????????????????????????????????????? bc no matter what the answer is going to be project 2!!!!!!!!!! what the fuck!!!!!!!!!!!
#speculation nation#like i Get that i need the practice regardless but COME ON at least make it a bit more variable!!!!!!#i have to write a whole summary of my findings that all boil down to 'well project 2 is mandated so uhh yea they gotta do that one'#i keep being like. 'maybe im reading it wrong? maybe it's not actually mandated?'#like if it's referring to something else that the project addresses#but no. it refers to the project. says it's entirely new. and mandated by the FDA.#FUCK dude i dont want to write a report that ultimately just says 'project 2 is mandated so Uhhh that one lol'#like project 2 is definitely better than the other two but project 3 is still the best. come on.#ughhh i finished the weighted scoring model at least. so i just gotta make the report.#i can definitely finish in time. im just frustrated by how pointless this all feels.
2 notes
·
View notes
Text

#Is Meticore safe- Are there any no side effects --#Meticore has been taken by thousands of folks with no reported side effects.#Click here to purchase Weight loose Meticore product :-#https://www.digistore24.com/redir/348520/Ameshram5/#And Meticore is a lot safer than starvation diets or hours of high intensity cardio at the gym#because you#are restoring your body's core temperature rather than disrupting it further. Addressing low core#temperature is the single most important thing you can do right now for a turbo-charged metabolism and#long-lasting results now and into old age.#Meticore is safer than your daily multivitamin. It has natural ingredients and they're extremely high quality#manufactured at an FDA-inspected#state-of-the-art facility#it's on the latest equipment and then on top of#that they're put through additional third-party inspections and quality control so you can rest assured that#Meticore is safe.#What results can I reasonably expect?#When you start Meticore be prepared for some big changes.#As your core temperature is addressed and metabolism is boosted#so you can expect stubborn fat to#decrease from all over your body. You can expect your skin to glow and feel plump and fresh. Your hair#will get silkier and your joints pain will ease.#Now#of course#the point is everyone is different. Everyone has slightly different body chemistry#so it's#difficult to say for sure which benefit you might experience first.#The best way to find out is to claim your own supply. Grab one of the three packages below and just give#it a shot. With our 60-day money-back guarantee#you can feel totally safe doing that.#Most folks are surprised because they have no idea how much their metabolism has been ruining their
3 notes
·
View notes
Text


In 1988, ACT UP protested the FDA withholding HIV treatment due to requiring unethical double-blind studies of medication they already knew worked.
In 2024, trans activists protested promoters of an NHS-funded report requiring unethical double-blind studies of medication they already knew worked.
21K notes
·
View notes
Text
A type of flu virus that used to sicken people every year hasn't been spotted anywhere on Earth since March 2020. As such, experts have advised that the apparently extinct viruses be removed from next year's flu vaccines.
The now-extinct viruses were a branch of the influenza B family tree known as the Yamagata lineage. Scientists first reported the apparent disappearance of Yamagata viruses in 2021. At that time, experts speculated that precautions taken to stop the spread of COVID-19 — such as masking and social distancing — had not only driven the overall number of flu cases to historic lows but may have completely snuffed out this type of flu virus.
Continue Reading.
20K notes
·
View notes
Text
Looking for a clinical study design in North Carolina - Durham to Raleigh, Research Triangle Park? You have come to the right place. We provide development and regulatory support of complete clinical development programs for an asset, innovative design of clinical studies, gap analysis of preclinical and clinical programs, and selection of appropriate populations for the studies. For more information, you can visit our website.
#clinical pharmacology boston-cambridge#pharmacometrics new jersey - princeton#regulatory strategy boston-cambridge#fda new york - new york city#clinical study protocol san diego#clinical study report san diego#modeling and simulation m&s north carolina#research triangle park
0 notes
Text
#IRB GPT#Institutional Review Board#protocol checker#GPT-4 technology#compliance#human subjects protection#The Belmont Report#The Common Rule#HIPAA Privacy Rule#FDA Regulations#HHS Regulations#OHRP Guidelines#FERPA#COPPA#NIH Guidelines#science and law#advisory role#compliance explanation#narrow AI#general AI#protocol writing#efficiency#hypothetical scenarios#fake protocols#Dietary Habits and Academic Performance#parental consent#child assent#privacy concerns#potential bias#impact on participants
1 note
·
View note
Text
Understanding the Role of the UK Responsible Person: Your Key to Compliance
One of the most significant consequences of Brexit for businesses placing products on the UK market is the emergence of the UK Responsible Person (UKRP). Similar to the European Authorized Representative (EAR) under the EU legislation, a UKRP acts as the point of contact for regulatory authorities and consumers. They ensure compliance with UK laws and regulations, taking on responsibilities formerly carried out by the EAR.
#MHRA Registration#US FDA Registration#UKCA Marking#EUDAMED Registration#UKRP#European Authorized Representative#FSC#Clinical Evaluation Report#UK Responsible Person#EAR#Free Sales Certificate#Regulatory and Market Intelligence
0 notes
Text
2023 Updates for Clinical Research Associates and Clinical Research Monitors
Common clinical trial guidelines used for monitors are designed to ensure the safety and accuracy of the data collected. These guidelines help to make sure that all participants in the trial are treated fairly and ethically, as well as ensuring that the results of the trial will be useful for medical research.
One important guideline is that the monitor must be independent from both the sponsor and investigator. The monitor should have no interest in or influence on the study's outcome, and must have complete access to any documents or records related to conducting the trial. Additionally, they are responsible for ensuring that all protocols are followed correctly, data is correctly recorded and stored, and any adverse events or reactions reported accurately and promptly.
Another key guideline is that monitors must act in accordance with Good Clinical Practice (GCP) guidelines established by International Conference on Harmonization (ICH). GCP outlines procedures for clinical trials involving human subjects so that ethical practices can be maintained throughout a study. It covers many topics including informed consent, protocol review, quality assurance/monitoring, investigator qualification requirements, patient safety procedures, and data verification methods.
Additionally, monitors may use other standards such as The Code of Federal Regulations (CFR), which is used by US Food & Drug Administration (FDA) to regulate drugs; International Committee on Harmonization (ICH) E6R2 ethical guidelines; European Medicines Agency’s Guidelines on Good Clinical Practice (GCP); World Health Organization’s International Ethical Guidelines for Biomedical Research Involving Human Subjects; or local regulations specified by each country’s health ministry.
Overall, these guidelines help to ensure that monitors remain impartial during a clinical trial - this helps to protect participant safety as well as providing reliable data for researchers later down the line.
Clinical research monitors are responsible for ensuring the safety of participants in clinical trials and the accuracy of data collected. In 2023, there have been several updates to guidelines for clinical research monitors that they should be aware of.
The United States Food and Drug Administration (FDA) has released Clinical Trials Guidance Documents that provide advice on the conduct of clinical trials, good clinical practice, and human subject protection. These documents outline the standards that must be met in order to ensure a safe and ethical trial environment.
Clinical research associates (CRAs) play a key role in medical research, ensuring that clinical trials are conducted according to the highest standards of quality, safety and ethics. In light of this importance, the U.S. Food and Drug Administration (FDA) has recently released new guidelines for CRAs conducting clinical trials. These guidelines provide an important framework to ensure that all research is conducted responsibly and ethically while protecting participants’ rights and safety. The FDA’s new guidelines focus on three main areas: data security, participant monitoring protocol, and communication with sponsors.
First, the FDA has established stringent data security measures to protect trial participants’ information during all stages of the trial process. This includes measures such as encryption of sensitive data, physical access control systems for secure areas where information is stored or processed, and regular backups of critical data sets to prevent any potential losses due to cyber-attacks or system malfunctions.
Second, the FDA requires that participation by CRAs in clinical trials include appropriate monitoring protocols designed to minimize risks associated with various trial procedures. This may include frequent communication with study sponsors about changes in protocol or patient status; close observation of trial participants; review and approval of all research documents before their use; scheduling regular safety assessments; and maintaining accurate records of all activities associated with each trial phase.
Finally, CRAs must maintain open communication channels with sponsors throughout the duration of a clinical trial in order to promptly report any changes in protocol or patient status that may require further review or approval from sponsors. Additionally, CRAs need to be trained on how to effectively communicate any necessary updates or potential issues related to regulatory compliance so they can ensure effective oversight over the entire course of a study period.
The FDA's new clinical trial guidelines provide an essential reference point for CRAs responsible for conducting medical research safely and ethically while protecting participants' rights and well-being. With these comprehensive guidelines in place, CRAs now have an even greater responsibility than ever when it comes to ensuring the success of health-related studies around the world.
Electronic Systems, Electronic Records, and Electronic Signatures in Clinical Investigations: Questions and Answers 3/15/2023
Considerations for the Design and Conduct of Externally Controlled Trials for Drug and Biological Products 1/31/2023
Clinical Investigator Administrative Actions — Disqualification 12/01/2022
Acute Myeloid Leukemia: Developing Drugs and Biological Products for Treatment 10/17/2022
Tissue Agnostic Drug Development in Oncology 10/17/2022
Characterizing, Collecting, and Reporting Immune-Mediated Adverse Reactions in Cancer Immunotherapeutic Clinical Trials 10/17/2022
Ethical Considerations for Clinical Investigations of Medical Products Involving Children 09/23/2022
Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drug and Biological Products 09/08/2022
We must always review the Handbook for Good Clinical Research Practice (GCP), which provides guidance on implementation of GCP standards. Additionally, the International Council for Harmonisation (ICH) has published Efficacy Guidelines which address design, conduct, safety and reporting of clinical trials.
2023 Good Clinical Practice Guidelines for Clinical Research Associates:
Clinical research associates must stay up-to-date on the latest clinical research regulations, guidance documents, and technology advancements in order to ensure ethical and compliant clinical trial management.
Clinical research associates must establish effective communication with all members of the research team to facilitate the exchange of information regarding study updates, timelines, and protocols.
Clinical research associates are responsible for performing accurate data entry into relevant databases or case report forms (CRFs) as part of their role in documenting results from clinical trials.
Clinical research associates must ensure that informed consent is obtained from all participants in accordance with local regulations and international ethical standards.
Clinical research associates must be knowledgeable about relevant In Vitro Diagnostic (IVD) device regulations and requirements for providing evidence of conformity, accuracy, and effectiveness prior to use in a study.
Clinical research associates should create detailed visit plans for each participant in order to maximize the efficiency of visits to investigator sites during a study without compromising data quality or patient safety.
Clinical research associates should conduct regular quality assurance (QA) activities such as source document verification (SDV), query resolution, audit trails, monitoring reports review, reconciliation activities etc., ensuring data accuracy throughout the course of a study period.
During audits or inspections conducted by regulatory authorities or ethics committees, clinical research associates must be prepared to present comprehensive documentation demonstrating compliance with GCP principles and local regulations governing clinical trial conduct.
The European Medicines Agency (EMA) has also released a Clinical Trials Regulation which harmonises processes for assessment and supervision of clinical trials throughout the EU. This regulation outlines requirements to ensure patient safety during a trial as well as evaluation procedures for new drugs or treatments being tested in a trial setting. Finally, The EQUATOR Network provides study protocols such as SPIRIT and PRISMA-P; diagnostic/prognostic studies such as STARD and TRIPOD; case reports such as CARE; extensions; clinical practice guidelines such as AGREE; all aimed at enhancing quality and transparency in health research publications.
In 2022, the US Food and Drug Administration (FDA) released new clinical trial guidelines that emphasize patient safety. The guidelines mandate that all clinical trials must adhere to a rigorous set of standards in order to ensure patient safety and efficacy.
The new guidelines require research teams to obtain written informed consent from participants prior to initiating any study activity. Abuse of animals is prohibited, and investigators are expected to use only those treatments that have shown potential benefit in animal studies. Additionally, researchers must report any adverse events or reactions during the course of the trial and ensure proper follow up care for affected individuals.
Furthermore, the FDA requires that research teams perform rigorous safety monitoring throughout the course of the trial. Regular data analyses and reviews must be conducted to identify potential risks and unexpected results, which must be reported in real time. Additionally, the FDA requires research teams to implement a system for tracking participant adherence to protocols, including collecting data on missed doses, changes in medication regimens, and other protocol violations.
The FDA also mandates more frequent reporting of results throughout the course of clinical trials. They require researchers to share interim results with stakeholders every six months or whenever significant changes occur in study design or purpose. These reports should include key findings as well as basic information about participant demographics and outcomes associated with each treatment arm.
Finally, the FDA has increased their emphasis on transparency by requiring researchers to disclose detailed information regarding sponsoring organizations and conflicts of interest associated with each study before it begins. This includes information related to payments made by sponsors as well as nonmonetary benefits received by investigators or other individuals associated with the trial.
By 2023, additional provisions will be added to these regulations including enhanced requirements related to diversity among participants; strengthened criteria for evaluating ethical considerations such as protection from harm; expanded definitions related to economic conflict-of-interest disclosure; greater emphasis on appropriate risk/benefit ratios; improved reporting of results utilizing standardized metrics; increased focus on study protocol adherence; enhanced data sharing practices; clear criteria for determining when further review is needed due health concerns; specified mechanisms for measuring patient quality-of-life outcomes; increased accountability through stronger recordkeeping systems; enhanced guidance around informed consent forms; improved methods for monitoring compliance; greater attention paid towards reviewing unpublished manuscripts related to clinical trials; expansion of proposed preventative measures targeting financial misconduct issues such as fraud detection systems; improved oversight mechanisms using Artificial Intelligence technologies such as natural language processing (NLP); and additional efforts aimed at improving public understanding around clinical trials through better communication strategies between sponsors and patients alike.
Stay up to date on clinical trials and your annual ICH GCP certification through one of the most comprehensive courses in the industry.
#fda guidelines for clinical trials pdf#clinical trial compensation guidelines#ich clinical trial guidelines#fda guidelines for clinical trials#ich guidelines for clinical trials#ich guidelines clinical trials#abpi clinical trial compensation guidelines#clinical trial advertising guidelines#clinical trial agreement negotiation guidelines#clinical trial archiving guidelines#clinical trial guidelines#clinical trial monitoring guidelines#clinical trial protocol guidelines#clinical trial reporting guidelines#clinical trials in australia guidelines#clinical trials medical devices guidelines#clinical trials pregnancy guidelines#compensation for clinical trial subjects as per ethical guidelines#consort guidelines for clinical trials#contemporary clinical trials author guidelines#data entry guidelines clinical trials#ema guidelines clinical trials#ema guidelines for clinical trials#ema guidelines for clinical trials pdf#ethical guidelines for clinical trials#eudralex volume 10 clinical trials guidelines#fda guidelines for clinical trials ppt#fda guidelines for monitoring clinical trials#first in human clinical trials guidelines fda#gcp guidelines for clinical trials
0 notes
Text
Additional types of cancer reported in people with breast implants, FDA says
Additional types of cancer reported in people with breast implants, FDA says
2022-09-09 08:46:06
The FDA announced Thursday that although it believes that occurrences of squamous cell carcinoma and various lymphomas in the capsule around breast implants may be rare, health care providers and people who have or are considering breast implants should be aware of these cases — and report them or any other cancers found around the implant to the agency.
These various…

View On WordPress
0 notes
Text

#Is Meticore safe- Are there any no side effects --#Meticore has been taken by thousands of folks with no reported side effects.#Click here to purchase Weight loose Meticore product :-#https://www.digistore24.com/redir/348520/Ameshram5/#And Meticore is a lot safer than starvation diets or hours of high intensity cardio at the gym#because you#are restoring your body's core temperature rather than disrupting it further. Addressing low core#temperature is the single most important thing you can do right now for a turbo-charged metabolism and#long-lasting results now and into old age.#Meticore is safer than your daily multivitamin. It has natural ingredients and they're extremely high quality#manufactured at an FDA-inspected#state-of-the-art facility#it's on the latest equipment and then on top of#that they're put through additional third-party inspections and quality control so you can rest assured that#Meticore is safe.#What results can I reasonably expect?#When you start Meticore be prepared for some big changes.#As your core temperature is addressed and metabolism is boosted#so you can expect stubborn fat to#decrease from all over your body. You can expect your skin to glow and feel plump and fresh. Your hair#will get silkier and your joints pain will ease.#Now#of course#the point is everyone is different. Everyone has slightly different body chemistry#so it's#difficult to say for sure which benefit you might experience first.#The best way to find out is to claim your own supply. Grab one of the three packages below and just give#it a shot. With our 60-day money-back guarantee#you can feel totally safe doing that.#Weight Loose
4 notes
·
View notes
Text
A2 Milk Jumps Before Halt on FDA Nod News, Denies Report
A2 Milk Jumps Before Halt on FDA Nod News, Denies Report
(Reuters) – Shares of New Zealand’s a2 Milk Co Ltd jumped more than 12% before trading in the stock was halted, after local media reported that the dairy company was close to winning an approval to sell baby formula in the United States.
A2 dismissed the report. The company had in May confirmed an application to the U.S. Food and Drug Administration (FDA) seeking permission to supply baby food…

View On WordPress
#Australia#Collections: Business#denies#FDA#halt#Investing News#jumps#milk#New Zealand#News#nod#report#Reuters#United States
0 notes
Text
The Best News of Last Week - March 18
1. FDA to Finally Outlaw Soda Ingredient Prohibited Around The World

An ingredient once commonly used in citrus-flavored sodas to keep the tangy taste mixed thoroughly through the beverage could finally be banned for good across the US. BVO, or brominated vegetable oil, is already banned in many countries, including India, Japan, and nations of the European Union, and was outlawed in the state of California in October 2022.
2. AI makes breakthrough discovery in battle to cure prostate cancer
Scientists have used AI to reveal a new form of aggressive prostate cancer which could revolutionise how the disease is diagnosed and treated.
A Cancer Research UK-funded study found prostate cancer, which affects one in eight men in their lifetime, includes two subtypes. It is hoped the findings could save thousands of lives in future and revolutionise how the cancer is diagnosed and treated.
3. “Inverse vaccine” shows potential to treat multiple sclerosis and other autoimmune diseases
A new type of vaccine developed by researchers at the University of Chicago’s Pritzker School of Molecular Engineering (PME) has shown in the lab setting that it can completely reverse autoimmune diseases like multiple sclerosis and type 1 diabetes — all without shutting down the rest of the immune system.
4. Paris 2024 Olympics makes history with unprecedented full gender parity

In a historic move, the International Olympic Committee (IOC) has distributed equal quotas for female and male athletes for the upcoming Olympic Games in Paris 2024. It is the first time The Olympics will have full gender parity and is a significant milestone in the pursuit of equal representation and opportunities for women in sports.
Biased media coverage lead girls and boys to abandon sports.
5. Restored coral reefs can grow as fast as healthy reefs in just 4 years, new research shows

Planting new coral in degraded reefs can lead to rapid recovery – with restored reefs growing as fast as healthy reefs after just four years. Researchers studied these reefs to assess whether coral restoration can bring back the important ecosystem functions of a healthy reef.
“The speed of recovery we saw is incredible,” said lead author Dr Ines Lange, from the University of Exeter.
6. EU regulators pass the planet's first sweeping AI regulations

The EU is banning practices that it believes will threaten citizens' rights. "Biometric categorization systems based on sensitive characteristics" will be outlawed, as will the "untargeted scraping" of images of faces from CCTV footage and the web to create facial recognition databases.
Other applications that will be banned include social scoring; emotion recognition in schools and workplaces; and "AI that manipulates human behavior or exploits people’s vulnerabilities."
7. Global child deaths reach historic low in 2022 – UN report

The number of children who died before their fifth birthday has reached a historic low, dropping to 4.9 million in 2022.
The report reveals that more children are surviving today than ever before, with the global under-5 mortality rate declining by 51 per cent since 2000.
---
That's it for this week :)
This newsletter will always be free. If you liked this post you can support me with a small kofi donation here:
Buy me a coffee ❤️
Also don’t forget to reblog this post with your friends.
732 notes
·
View notes