#phase 1 clinical trials
Text

Amazing indeed! Read the whole thread.
15 notes
·
View notes
Text
Clinical trials are a crucial step in the creation of new medications. Prior to being made available to the general public, these trials are carried out to evaluate the efficacy and safety of novel medications. There are four phases in the clinical trial process, and each has its own unique objectives and specifications. The four stages of clinical trials and what they include will be covered in this article.

Phase 1 Clinical Trials
The initial stage of evaluating a new medicine on humans is called a phase 1 clinical study. This stage's objectives are to evaluate the drug's safety and spot any potential negative effects. Phase 1 studies typically comprise a small number of healthy volunteers, though, in some circumstances, patients with the condition the medicine is meant to treat may also be included.
A small number of individuals receive a modest dose of the medication during a phase 1 clinical trial. For any unfavorable reactions or side effects, the participants are closely watched. If the medication is determined to be safe, the dose is increased and the trial is continued with more individuals.
Phase 2 Clinical Trials
Phase 1 drug safety testing should be completed before moving on to phase 2. This phase's objective is to evaluate the medication's efficacy in managing the condition for which it was intended. Phase 2 trials contain a bigger participant pool, usually between 100 and 500 people.
To find the best dosage for treatment, participants in a phase 2 trial are administered the medicine at various doses. There is also a control group in the trial that receives a placebo, a material that resembles a medicine but has no actual active components. To ascertain the drug's efficacy, the trial's outcomes are then contrasted between the two groups.
Phase 3 Clinical Trials
Phase 3 testing should begin when a drug's effectiveness in phase 2 has been established. This phase's objectives are to verify the drug's efficacy and obtain further data on its safety. Phase 3 trials typically involve several hundred to several thousand more subjects.
In a phase 3 trial, the medicine is contrasted with currently available therapies for the illness it is intended to treat. This aids in figuring out whether the new medication is more efficient or has fewer adverse effects than current therapies. To confirm that the medication is safe for use by the general public, other safety information is also gathered during the study.
Phase 4 Clinical Trials
A medication enters phase 4 clinical trials after being given regulatory agency approval for general use. This phase's objective is to compile more data on the medication's long-term safety and efficacy. Phase 4 trials can take several years to complete and generally involve thousands of people.
The medication is watched during a phase 4 trial for any potential long-term negative effects or problems. The trial also assists in identifying any unanticipated uses or advantages of the medication. The medicine's labeling, prescribing information, and upcoming research on the drug will all be updated using this information.
Conclusion
The process of developing new drugs must include clinical trials. Before new medications are made available to the general population, they assist in ensuring their efficacy and safety. There are four phases in the clinical trial process, and each has its own unique objectives and specifications. Phase 1 studies determine the safety of the drug, phase 2 trials demonstrate the drug's efficacy, phase 3 trials validate the drug's efficacy and safety, and phase 4 trials collect long-term safety and efficacy data. We may use this knowledge to make educated choices about the medications we take and have confidence that they are both safe and effective.
0 notes
Text
Uncovering the Role of Phase I Studies in Drug Development

Introduction
In drug development, a phase I study is the initial period of testing in humans. This stage is where you can make sure that your new compound is safe and effective enough to warrant further investigation. While it's important to have an effective Phase I program in place, there are several other aspects of clinical trial design and management that should be considered as well. These include designing an optimal dose for Phase II studies and ensuring the safety of your candidate drug.
Designing a clinical trial program for your new compound
Before you begin your early clinical trial program, you need to ensure that your compound is safe and well-tolerated. This can be accomplished with a phase I study. A phase I study is the first step in drug development and helps determine if your drug is safe and effective for humans. In addition to providing insights into whether or not further clinical trials should be pursued, it also provides information about how best to design those trials based on how people respond when given varying amounts of the new drug.
The goal of the phase I clinical trial is to collect information about what happens when an experimental drug is administered at different doses over a range of time periods (e.g., 2 hours versus 4 hours). It's important that each subject receive both high and low doses so that scientists can see how much variation there might be between people taking different amounts of this new compound. The most common way this occurs is through giving participants escalating doses (first small initial dose followed by larger ones as time passes) or by giving them fixed doses every few days until they reach their maximal tolerated dose (MTD), which involves gradually increasing their daily intake until they have taken enough medicine for several days in a row without showing any adverse side effects such as nausea or diarrhea; however, there are other ways as well!
Selecting the optimal dose for Phase II
Phase II studies are designed to assess the efficacy and safety of a compound. A dose escalation study is typically done in this phase, because it is important to determine the effective dose of a drug before proceeding with expensive Phase III trials.
Selecting the optimal dose for Phase II studies can be challenging if you do not have any relevant data from your previous studies (i.e., Phase I). In such cases, you may need to consider using an established approach such as Bayesian statistics or adaptive randomization technique.
Optimizing your clinical program and trial design
As the development of a drug proceeds, it is important to optimize your clinical program and trial design. This will help ensure that you get the most out of your studies and are able to move on to later stage trials in a timely manner.
When planning and conducting Phase I studies, consider:
The trial design and study population
Whether or not the study is scalable for use in later-stage trials
How feasible, ethical, and sustainable will this study be?

Determining the safety of your candidate drug
Phase I studies are the first step in a drug development process. They are designed to test for safety, not efficacy. Phase II studies determine whether a drug has any potential to be effective. Phase III studies test for efficacy and side effects in human subjects.
Phase II studies usually include 100-300 participants; phase III typically includes 2,000-3,000 participants. These phases of testing are very expensive and take several years to complete; they must also be approved by regulators before they can begin testing on humans.
Identifying potential toxicities
In a phase I study, you are given a small dose of your molecule and its effects on the body are observed. If the molecule is toxic and has adverse effects, you will have to do more research and development to come up with a safer product. However, it may be that your drug is not toxic at all, or maybe only one pathway in the body gets affected by it. In either case, if you cannot identify how your compound works and what other pathways might be affected by it in phase I, then any additional work will simply be more expensive in later stages when you need more patients taking larger doses of your drug.
Determining the pharmacokinetic profile of your compound
Pharmacokinetics is the study of how a drug is absorbed, distributed, metabolized, and excreted by the body. The pharmacokinetic profile of your compound will determine whether it has a safe and effective dose range.
These studies are typically done in cells or animals so that you can get answers about how quickly your drug will be broken down by enzymes in the body.
Collecting and analyzing data on your compound's metabolism, disposition, and excretion
In order to correctly interpret the results of your study, you will need to understand the difference between "metabolism," "disposition," and "excretion." If a compound is metabolized into more than one product, then those products are considered metabolites. The sum of all metabolites that arise from a single compound is known as its metabolic profile. The rate at which metabolites are formed from their parent drug depends on the enzyme(s) involved in this process (known as an enzyme-mediated reaction). You can use your Phase I studies to identify enzymes that may be involved in metabolism, including those responsible for making hydroxylated metabolites (e.g., 2-OH form).
Effective phase I studies can ensure that you move into later stages of drug development with confidence.
Phase I studies are essential to drug development. They're small in size, which means they can be completed quickly, and they focus on establishing safety. They're also the first step in clinical trials and don't require many subjects to complete.
Conclusion
The success of a phase I study is not only dependent on the science that goes into designing it, but also on the planning and execution by those involved in its execution. The information gathered during this pivotal stage in drug development can be used to improve future clinical trials and help scientists make better decisions about how their compounds should be developed.
0 notes
Text

#early phase clinical research#early phase studies#early phase clinical trials#early phase study#early phase 1 clinical trial#early stage clinical trials
0 notes
Text
I saw some mentions of rabies going around again and have no clue what's set it off this time, but given recent scientific developments I want to revisit the idea of curing symptomatic rabies.
First things first: there is still no practical way to do this. The famous Milwaukee Protocol fails far more frequently than it succeeds, and even the successes are not making it out in anything like a normal state. It's been argued that it should no longer be considered a valid treatment [1] due to these issues; any continued use is because there's literally nothing else on the table.
However. There are now two separate studies showing it's possible to cure rabies in mice after the onset of symptoms. The lengths you have to go to in order to pull this off are drastic, to put it mildly, and couldn't really be adapted to humans even if you wanted to. But proof of concept is now on the board.
long post under the cut, warnings for animal experimentation and animal death. full bibliography at the end and first mention of each source links to paper.
Quick recap - rabies is a viral disease of mammals usually transmitted through the saliva of an infected animal. From a contaminated bite wound, it propagates slowly for anywhere from days to months until it reaches the central nervous system (CNS). Post-exposure vaccination can head it off during this phase, but once it reaches the CNS and neurological symptoms appear it's game over. There will typically be a prodromal phase where the animal doesn't act right - out at the wrong time of day, disoriented, abnormally friendly, etc. This will then progress to the furious (stereotypical "mad dog" disease) and/or paralytic phases, with death eventually caused by either seizures or paralysis of the muscles needed for breathing.
That's the course we're familiar with in larger animals. Mice, though, are fragile little creatures with fast metabolisms.
In the first study's rabies infection model, lab mice show rabies virus in the spinal cord by day 4 after infection and in the brain by day 5. Weight loss and slower movement start by day 7, paralysis starting from the hind limbs from day 8 on, and if not euthanized first they're dead by day 10-13. [2]
This study (fittingly conducted at the Institut Pasteur) had two human monoclonal antibodies, and wanted to see if there was any possibility they could be used to cure rabies after what we think of as the point of no return.
Injecting the antibodies into muscle saved some mice if done at days 2 or 4, and none if done later, even at high doses of 20 milligrams per kilogram of body weight of each. Conclusion: targeting the virus out in the rest of the body is no use if it's already replicating in the CNS.
Getting a drug past the blood-brain barrier is, to use a highly technical term, really fucking hard. It's the sort of problem that even the best-funded labs and biggest companies in the world routinely fail at. And that's for small molecule drugs, which are puny compared to antibodies.
But this isn't drug development for a clinical trial. This is a very, very early proof-of-concept attempt, which means you're willing to ignore practicality to see if this idea is even remotely workable. So you can do things like brute force the issue by cutting through the skull to implant a microinfusion pump, which lets you deliver the antibodies directly into the normally-protected space around the brain. Combine this with the normal injections, and you can treat both the CNS and the rest of the body at the same time. Here's a survival graph of treated mice. X axis is days, Y axis is percentage of mice in that group still alive.
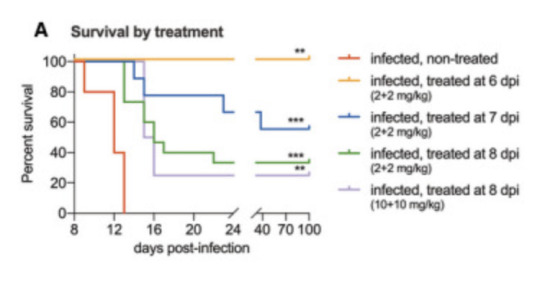
Figure 2A from reference 2, accessed February 2024
The fact that the blue, green, and purple lines did anything other than sink horribly to zero is unheard of. When the combination treatment was started at day 6, 100% of the mice survived. Started at day 7 (prodromal phase), 5 out of 9 mice recovered and survived. Started at day 8 (solidly symptomatic, paralysis already starting to set in), 5 of 15 mice recovered and survived. And when they say "survived", they kept these mice all the way to day 100 to make sure. Some of them had permanent minor paralysis but largely they were back to being normal mice doing normal mouse things. So, success, but by pretty extreme means.
Enter the second paper [3]. This was a different approach using a single human monoclonal antibody against Australian bat lyssavirus (ABLV - closely related to rabies, similar symptoms in humans) to try for a cure without needing to deliver treatments directly into the CNS. They also made a luminescent version of ABLV that let them directly image viral activity, so they could see both where the virus was replicating and how much there was in a live mouse.

Figure 1 from reference 3, accessed February 2024
Mice infected with ABLV start showing symptoms around day 8. You can see in the figure that at day 3 there's viral replication in the foot at the site of infection, which has shifted into the spine and brain by day 10. So what happens if you give one of these doomed mice one single injection of the antibody into the body?
Done at day 3, the virus doesn't make it to the brain until day 14, and while disease does set in after that around 30% of the mice survive. Days 5 and 7 are much more interesting. Those mice still develop symptoms at day 8, but the imaging shows the amount of virus in their spines and brains never gets anywhere near the levels seen in untreated controls, and within days it starts to decrease. Around 80% of day 5 and 100% of day 7 mice survive.
Okay, sure, you can stop another lyssavirus, but technically you did start treatment before symptoms appeared. What about symptomatic rabies?
The rodent-adapted rabies strain CVS-11 starts causing symptoms as early as day 3 after infection, and untreated mice die between days 8 and 11. The same single dose of antibody saved 67% of mice treated on day 5 and 50% of mice treated on day 7. Without making the luminescent version of the virus there's no real-time imaging of the infection, but you can still track symptoms.
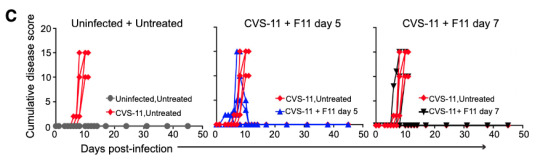
Figure 2 from reference 3, accessed February 2024. CVS-11 is the name of the rodent rabies strain and F11 is the name of the antibody.
Disease score is a combination of several metrics including things like whether the mice are behaving normally and whether they show signs of paralysis. In untreated mice it goes up and up, and then they die. If one of those lines starts coming back down and continues past day 10 or so, that's a mouse that recovered. The success rate isn't as good as against ABLV, but again, this is a rabies strain specifically adapted to rodents and treatment wasn't started until it was well-established in the CNS.
So how on earth is this happening? The antibody neutralizes both ABLV and rabies really well in a test tube, but we've already established that there's no way a huge lumbering antibody is making it past the blood-brain barrier without serious help. Something about the immune response is clearly making it in there though. And it turns out that if you start trying this cure in mice missing various parts of their immune systems, mice without CD4+ T cells don't survive even with the treatment. By contrast mice without CD8+ T cells take longer to work through the infection, but they eventually manage it and are immune to reinfection afterwards.
To grossly oversimplify the immune system here, CD4+ are mature helper T cells, which work mostly by activating other immune cells like macrophages (white blood cells) and CD8+ T cells (killer T cells) against a threat.
Normally, T cells are also kept out by the blood-brain barrier, but we know that in certain specific cases including viral infection they can pass it to migrate into the brain. In the brains of the infected mice for which antibody treatment either wasn't given or didn't work, you can find a roughly even mix of CD8+ and CD4+ T cells along with a whole lot of viral RNA. But in the brains of those successfully fighting off the infection, there's less viral RNA and the cells are almost exclusively CD4+. So the antibody doesn't work by neutralizing the virus directly - something about it is activating the animal's own immune system in a way that gives it a fighting chance.
Again, neither of these proof of concept treatments is really workable yet as a real world cure. The first one is almost hilariously overkill and still has a pretty good chance of failure. The second is less invasive but careful sequencing still shows both low-level viral replication and signs of immune response in the brains of the survivors even at day 139, so it may not be truly clearing the virus so much as trading a death sentence for life with a low-level chronic infection. But now we know that 1. curing rabies after symptoms begin is at least theoretically possible, and 2. we have some clues as to mechanisms to investigate further.
Not today. Not tomorrow. But maybe not never, either.
References:
Zeiler, F. A., & Jackson, A. C. (2016). Critical appraisal of the Milwaukee protocol for rabies: this failed approach should be abandoned. Canadian Journal of Neurological Sciences, 43(1), 44-51.
de Melo, G. D., Sonthonnax, F., Lepousez, G., Jouvion, G., Minola, A., Zatta, F., ... & Bourhy, H. (2020). A combination of two human monoclonal antibodies cures symptomatic rabies. EMBO molecular medicine, 12(11), e12628.
Mastraccio, K. E., Huaman, C., Coggins, S. A. A., Clouse, C., Rader, M., Yan, L., ... & Schaefer, B. C. (2023). mAb therapy controls CNS‐resident lyssavirus infection via a CD4 T cell‐dependent mechanism. EMBO Molecular Medicine, 15(10), e16394.
244 notes
·
View notes
Text
1. Girl with incurable cancer recovers after pioneering treatment
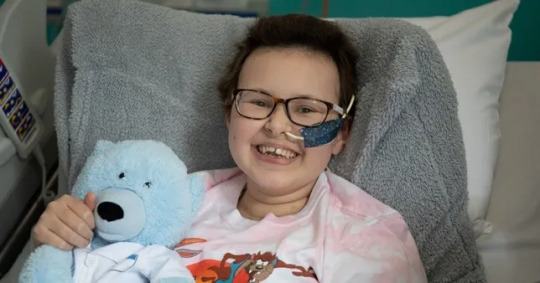
A girl’s incurable cancer has been cleared from her body after what scientists have described as the most sophisticated cell engineering to date. Alyssa, whose family do not wish to give their surname, was diagnosed with T-cell acute lymphoblastic leukaemia in May 2021.
Scientists at Great Ormond Street Hospital for Children in London gave her pre-manufactured cells edited using new technology to allow them to hunt down and destroy cancerous T-cells without attacking each other. Less than a month after being given the treatment, she was in remission, and was able to have a second bone marrow transplant.
Can I get a fuck cancer?
2. The UK has made gigabit internet a legal requirement for new homes

Updated regulations require new properties to be built with gigabit broadband connections and make it easier to install into existing blocks of flats across the UK. Connection costs will be capped at £2,000 per home, and developers must still install gigabit-ready infrastructure (including ducts, chambers, and termination points) and the fastest-available connection if they’re unable to secure a gigabit connection within the cost cap
3. US cancer death rate falls 33% since 1991

The rate of people dying from cancer in the United States has continuously declined over the past three decades, according to a new report from the American Cancer Society.
The US cancer death rate has fallen 33% since 1991, which corresponds to an estimated 3.8 million deaths averted, according to the report, published Thursday in CA: A Cancer Journal for Clinicians. Partly due to advances in treatment, early detection and less smoking, report says
. . .
Thank you for following. You can support this newsletter for less than a price of a cup of coffee:
Support with a coffee ❤️
Let’s continue with the good news…
. . .
4. Lab-grown retinal eye cells make successful connections, open door for clinical trials to treat blindness

Retinal cells grown from stem cells can reach out and connect with neighbors, according to a new study, completing a “handshake” that may show the cells are ready for trials in humans with degenerative eye disorders.
Over a decade ago, researchers from the University of Wisconsin–Madison developed a way to grow organized clusters of cells, called organoids, that resemble the retina, the light-sensitive tissue at the back of the eye. They coaxed human skin cells reprogrammed to act as stem cells to develop into layers of several types of retinal cells that sense light and ultimately transmit what we see to the brain.
5. The ozone layer is on track to recover in the next 40 years, the United Nations says

The Earth's ozone layer is on its way to recovering, thanks to decades of work to get rid of ozone-damaging chemicals, a panel of international experts backed by the United Nations has found.
The ozone layer serves an important function for living things on Earth. This shield in the stratosphere protects humans and the environment from harmful levels of the sun's ultraviolet radiation. In the latest report on the progress of the Montreal Protocol, the U.N.-backed panel confirmed that nearly 99% of banned ozone-depleting substances have been phased out.
6. Uganda declares an end to Ebola outbreak

The Ugandan government has declared an end to its Ebola outbreak, less than four months after cases were first reported. Since 20 September, 56 people have died from the virus, which is spread through body fluids, and there have been 142 confirmed infections.
The country has reported no new infections in more than 42 days – twice the maximum incubation period of the virus, a World Health Organization benchmark for a country to be declared Ebola-free.
7. Doggy ‘daycare’ bus in Alaska goes viral on TikTok
Check them out here:
- - -
That's it for this week. If you liked this post you can support this newsletter with a small kofi donation:
Buy me a coffee ❤️
Subscribe for more weekly wholesome news...
469 notes
·
View notes
Text
What’s a CDD?
Easy.
DID and OSDD 1.
Sources?
The authors of the ToSD
People without access to a proper library really shouldn’t be in syscourse, ffs, why are we arguing about this? >:[ stop it
All sources under the cut
According to the Theory of Structural Dissociation of the Personality, trauma-related disorders can be described on a continuum from simple PTSD, to cPTSD, to more complex dissociative conditions such as DDNOS-1 and DID.
Resting‐state functional connectivity in patients with a complex PTSD or complex dissociative disorder before and after inpatient trauma treatment - Nijenhuis, Ellert R S
In the treatment of complex dissociative disorders (i.e., dissociative identity disorder [DID] and dissociative disorder not otherwise specified [DDNOS] it is often unclear if the patient will be capable of integrating the traumatic past).
TREATMENT STRATEGIES FOR COMPLEX DISSOCIATIVE DISORDERS - van der Hart, O
These complex disorders are considered to be extreme reactions to developmental trauma and require a specific treatment approach. They include Dissociative Identity Disorder (DID) and Other Specified Dissociative Disorder-Type 1 (OSDD-1; APA, 2013). OSDD includes a diverse range of four dissociative problems, but OSDD-1 is considered to be very much like DID with less distinct symptoms.
Assessing and Treating Complex Dissociative Disorders - Steele, van der Hart
Various studies found, among psychiatric inpatients, prevalence rates of 421% for these dissociative disorders as a whole and 17% for the most complex dissociative disorder, i.e., dissociative identity disorder (DID) (see Sar, 2011, for an overview). For the complex DSM-IV dissociative disorders, i.e., DID and dissociative disorder not otherwise specified (DDNOS, subtype 1b), as well as for other complex trauma-related disorders such as Complex PTSD, the standard of care is phase-oriented treatment consisting of...
The use of imagery in phase 1 treatment of clients with complex dissociative disorders - van der Hart, O
According to the current standard of care, the treatment of traumatic memories of patients with complex trauma-related disorders – including dissociative identity disorder (DID) and DSM-5 other specified dissociative disorder [OSDD] (DSM-IV dissociative disorder not otherwise specified [DDNOS]) – involves a phase-oriented treatment approach.
The treatment of traumatic memories in patients with complex
dissociative disorders - O. van der Hart, K. Steele, E. Nijenhuis
Other sources:
Chronic complex DD include dissociative identity disorder (DID) and the most common form of dissociative disorder not otherwise specified (DDNOS, type 1), now known as Other Specified Dissociative Disorders (OSDD, type 1).
Chronic complex dissociative disorders and borderline personality disorder: disorders of emotion dysregulation?
Once there are multiple ANPs and EPs then a complex dissociative disorder is in place, structural dissociation applies.
UNRESOLVED PROBLEMS IN THE THEORY OF STRUCTURAL DISSOCIATION
(reminder of the theory for context)

The term ‘complex dissociative disorder’ (CDD) used by some authors (see Dell, 2009) refers to the most severe dissociative conditions, that is, the diagnoses Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV/DSM-5 dissociative identity disorder (DID) and DSM-IV dissociative disorder not otherwise specified, subtype 1 (DDNOS-1)/DSM- 5 other specified dissociative disorders-1 (OSDD-1) (APA, 2000, 2013).
Negative affective responses to positive events and stimuli in patients with
complex dissociative disorders: a mixed-methods pilot study
DID and the closely related Other Specified Dissociative Disorders, example 1 (OSDD), where similar disturbances are observed without meeting the full clinical picture of DID, are commonly categorized as Complex Dissociative Disorders (CDD).
Group treatment for complex dissociative disorders: a randomized clinical trial
The participants were all diagnosed with complex dissociative disorders (CDD), that is, dissociative identity disorder (DID) and Dissociative disorder not otherwise specified (DDNOS).
The challenge of being present with yourself: Exploring the lived experience of individuals with complex dissociative disorders
Psychotic symptoms occur in some, but not all dissociative disorders. They are usually seen in dissociative identity disorder (DID) and dissociative disorder not otherwise specified type I (DDNOS-1, according to the DSM-IV), traditionally subsumed under the rubric of ‘complex’ dissociative disorders (Loewenstein, 1991).
Psychosis, Trauma and Dissociation Emerging Perspectives on Severe Psychopathology
There is a paucity of empirical data to assist clinicians in choosing interventions to use with patients with complex dissociative disorder (DD; i.e., dissociative identity disorder and dissociative disorder not otherwise specified) at different stages in treatment.
Treatment of complex dissociative disorders: A comparison of interventions reported by community therapists versus those recommended by experts.
This information resource outlines the rationale, response and outcomes required for recovery from complex trauma when there is also a significant dissociative presentation. In particular, when Dissociative Identity Disorder (DID), similar trauma-related complex dissociative conditions or Complex PTSD (as a result of severe, prolonged and repeated traumas - usually abuse - beginning in early childhood) is the most accurate primary psychiatric diagnosis.
ESTD Complex Trauma resulting in Dissociative Identity and similar Dissociative Post-Traumatic Conditions
Most people at the severe end of the dissociative spectrum, living with Dissociative Identity Disorder (DID) or Dissociative Disorder Not Otherwise Specified (DDNOS), have suffered severe, and often organised criminal trauma and abuse in childhood, against a background of disorganised attachment. Complex dissociative disorders including DID and DDNOS are widely understood as complex forms of post-traumatic stress disorder, where some combination of severe neglect, trauma, and abuse has occurred in childhood, against a background of disorganised attachment and across a child's developmental stages.
Medical aspects of recognising complex dissociative disorders
Complex dissociative disorders (CDD) include dissociative identity disorder (DID) and the most common other specified dissociative disorder (OSDD, type 1). One of the strongest predictors of CDD is antecedent trauma, particularly early childhood trauma.
A Schema Therapy Approach to Complex Dissociative Disorder in a Cross-Cultural Setting
43 notes
·
View notes
Text
Dear all, a news digest from an American contact.
Very concerning. The perpetrators evidently intend to do it all over again, based on exactly the same lies.
Scary respiratory virus lies.
Mask mandate lies.
Vaccine mandate lies (read between the lines of the Biden quote).
Lockdowns are an obvious component of the alleged public health emergency, which will be wholly fictitious. How else can they predict what’s going to happen, other than the same people running the scare?
Do not comply.
Best wishes
Mike
Morning Intelligence Report
COVID-19 and the Vaccines
By
Paul E Vallely, MG US Army (Ret)
August 27, 2023
COVID-19 and Vaccines could be the most significant fraud perpetrated on the world and the greatest transfer of wealth to the global elites and organizations like Pfizer. Do not fall into the Trap!
Inside information exposes that TSA and US Border Patrol will be moving back to 2020-era COVID-19 mandates and restrictions from mid-September through mid-October, including mask mandates on all flights. This is in addition to the confirmed mask-mandate reinstatement at Morris Brown College in Atlanta, GA, and Lionsgate Studios in Santa Monica, CA. Also, a school district in South Texas just outside San Antonio closed down temporarily due to an ‘uptick’ in COVID cases.
That same week, WarRoom’s Natalie Winters uncovered millions of dollars in funding, awarded primarily to the Department of Veterans Affairs and DoD, to ramp up testing and other COVID-19 related.
This was just a week after the NIH appointed Dr. Jeanne Marrazzo, a staunch advocate for masks, lockdowns, and vaccine mandates, as the replacement for Dr. Fauci.
To further the suggestion that another lockdown scare is in the forecast, on Tuesday, the US Department of Health and Human Services announced funding of $1.4 billion to “support the development of a new generation of tools and technologies to protect against COVID-19 for years to come” according to a press release.
Why a Veteran-Owned Freeze-Dried Beef Company Unabashedly Embraces an America First Worldview
“Project NextGen is a key part of the Biden-Harris Administration’s commitment to keeping people safe from COVID-19 variants,” said HHS Secretary Xavier Becerra. “These awards are a catalyst for the program – kickstarting efforts to more quickly develop vaccines and continue to ensure availability of effective treatments.”
Project NextGen, a $5 billion initiative led by ASPR’s Biomedical Advanced Research and Development Authority (BARDA) in partnership with the National Institute of Allergy and Infectious Diseases (NIAID), coordinates across the federal government and the private sector to advance innovative vaccines and therapeutics into clinical trials, regulatory review, and potential commercial availability for the American people. The project builds on a better understanding of COVID-19 – with HHS developing, using, and constantly re-evaluating the strengths and weaknesses of current vaccines and therapeutics for over three years.
Recipients of the awards include:
· $1 billion to four BARDA Clinical Trial partners to support vaccine Phase IIb clinical trial studies: ICON Government and Public Health Solutions, Inc. of Hinckley, Ohio; Pharm-Olam, LLC, of Houston, Texas; Technical Resources Intl (TRI), Inc, of Bethesda, Maryland; and Rho Federal Systems, Inc., Durham, North Carolina.
· $326 million to Regeneron to support the development of a next-generation monoclonal antibody for COVID-19 prevention.
· $100 million to Global Health Investment Corp. (GHIC), the non-profit organization managing the BARDA Ventures investment portfolio, to expand investments in new technologies that will accelerate responses in the future.
· $10 million to Johnson & Johnson Innovation (JLABS) for competition through Blue Knight, a BARDA-JLABS partnership.
11 notes
·
View notes
Note
Hello!
I thought about you. Just checking in :)
Well let's see...
I was supposed to start a new job but it requires an extensive background check that is taking FOREVER so I'm in a weird holding pattern where I can't get a full time job but also I'm not employed. This has been going on for (check watch) seven months.
After years of not buying a mandoline (kitchen slicer) because I know myself and know I'm injury prone especially in the kitchen, I finally got one and purposefully got the one with all the safety features and cut-proof gloves. I then immediately, literally the first time I used it, got a potato stuck on the blade and while trying to figure out why it was stuck it became very unstuck and I sliced about 90% of my fingertip off. I was standing next to the cut proof gloves when this happened - they don't work sitting on the counter. That was a 9 stitches trip to the ER. They just came out yesterday.
But! I swear to god the epilogue / sequel novella to Love Nor Money is going to come out on December 1st if it kills me. So I've been writing and this will serve as both a bonus story for the Love Nor Money crew as well as setting up a possible sequel for Nico and Jess should I ever get my life together to get around to it.
Also my dad has untreatable lung cancer and is now in a Phase 1 clinical trial and well that's a whole other thing.
So yeah, that's how I'm doing. If you were ever wondering why there's no fanfic moving from me this is all why.
9 notes
·
View notes
Text
0 notes
Text
#protocol writing in clinical research#protocol writing for acne vulgaris#writing an acne protocol#acne clinical research protocol writing#clinical trial protocol writing#drafting protocol for clinical trial#writing protocols for clinical trials#clinical trial protocol example#phase 1 clinical trial protocol template
0 notes
Text
Advancements in Phase 1 Clinical Trial Services in India.
Introduction:
India has emerged as a pivotal player in the global pharmaceutical and clinical research landscape, offering a conducive environment for conducting Phase 1 clinical trials. As the pharmaceutical industry continues to evolve, the emphasis on early-phase trials has gained significant momentum. This article explores the diverse range of Phase 1 clinical trial services available in India and highlights the country's growing prominence in this critical stage of drug development.

Key Features of Phase 1 Clinical Trial Services in India:
Regulatory Framework: India boasts a robust regulatory framework that ensures the ethical and scientific conduct of clinical trials. The Central Drugs Standard Control Organization (CDSCO) and the Drug Controller General of India (DCGI) play pivotal roles in overseeing and regulating clinical research activities.
Experienced Investigator Pools: The country is home to a vast pool of skilled and experienced investigators and clinical research professionals. These experts adhere to international quality standards and guidelines, ensuring the reliability and integrity of Phase 1 clinical trials conducted in India.
State-of-the-Art Infrastructure: Leading research institutions, hospitals, and specialized clinical research organizations (CROs) in India have invested significantly in cutting-edge infrastructure to facilitate Phase 1 trials. Well-equipped facilities and advanced technologies enhance the efficiency and accuracy of data collection and analysis.
Diverse Patient Populations: India's diverse population offers a unique advantage for pharmaceutical companies conducting Phase 1 trials. The inclusion of various ethnic groups and genetic diversities ensures a more comprehensive understanding of a drug's safety and efficacy across different demographics.
Cost-Effectiveness:
Conducting Phase 1 clinical trial services in India is often more cost-effective compared to many Western countries. This cost advantage does not compromise the quality of research but makes India an attractive destination for sponsors looking to optimize their research budgets.
Quick Regulatory Approvals: India's regulatory authorities have streamlined the approval process for clinical trials, ensuring that sponsors experience minimal delays in initiating Phase 1 studies. This swift approval process allows for faster recruitment of subjects and timely completion of trials.
International Collaboration:
Many global pharmaceutical companies and CROs collaborate with Indian research institutions and investigators for Phase 1 trials. This collaborative approach fosters knowledge exchange, bringing together the best practices from around the world.
Conclusion:
India's growing stature in the field of Phase 1 clinical trials is a testament to its commitment to fostering a research-friendly environment. The combination of a robust regulatory framework, experienced investigators, advanced infrastructure, diverse patient populations, cost-effectiveness, and quick regulatory approvals positions India as a key player in the global landscape of early-phase clinical research. As the pharmaceutical industry continues to evolve, India's contributions to Phase 1 clinical trials are likely to play a pivotal role in advancing drug development and improving global healthcare outcomes.
0 notes
Text

(copy-paste)
Chise. Senior Scientist / Vaccine Research & Development:
"So, not COVID related, however, this is REALLY exciting news. For the FIRST time ever, we have evidence that it IS possible to develop a functional immune response that can treat patients’ cancer based on Phase 2b trial results of an investigational personalized cancer vaccine!
Phase 2b KEYNOTE-942/mRNA-4157-P201 trial of mRNA-4157/V940, in combination with KEYTRUDA®, Merck's anti-PD-1 therapy, demonstrated a statistically significant and clinically meaningful improvement in the primary endpoint of recurrence-free survival (RFS) versus KEYTRUDA alone for the adjuvant treatment of patients with stage III/IV melanoma following complete resection.
Adjuvant treatment with mRNA-4157/V940 in combination with KEYTRUDA reduced the risk of recurrence or death by 44% compared with KEYTRUDA alone. These results are the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial.
Needless to say, that is ASTOUNDING. In the study, patients were randomly assigned to receive one of two treatments. One group was treated with the drug pembrolizumab, or Keytruda, an existing medication that releases the brake that the immune system normally has on attacking cancer cells, since cancer cells grow from the body’s own cells.
The other group received Keytruda and a personalized cancer vaccine using mRNA technology. All of the patients had surgery to remove their melanoma, and for the vaccine group, scientists biopsied and genetically sequenced those tumors, then identified nearly three dozen genetic, personalized tumor flags, in the form of mRNA, for each patient’s immune system to recognize.
These were then combined and injected in patients’ arms-in the same way that the COVID-19 vaccine delivered instructions to target the virus’ spike protein genes. Except in this case, the immune system was trained to target and destroy melanoma cells rather than a virus.
So, how exactly does this work? Personalized cancer vaccines are designed to prime the immune system so that a patient can generate a tailored antitumor response specific to their tumor mutation signature. mRNA-4157/V940 is designed to stimulate an immune response by generating specific T-cell responses based on the unique mutational signature of a patient's tumor.
mRNA-4157/V940 essentially consists of a single synthetic mRNA coding for up to 34 neoantigens that is designed and produced based on the unique mutational signature of the DNA sequence of the patient's tumor. Upon administration into the body, the algorithmically derived and RNA-encoded neoantigen sequences are endogenously translated and undergo natural cellular antigen processing and presentation, which is a key step in adaptive immunity.


ID: The key to the vaccine's success seems to be that it is tailored to each patient's tumor, allowing each patient to mount a precise and targeted response to their cancer. The flexibility of the mRNA technology makes that possible, as COVID-19 vaccine development demonstrated. It took about six weeks for scientists to generate each personalized mRNA cancer vaccine.
Patients will be followed for at least one more year, and possibly more after completing the treatment. Scientists will monitor how long- lasting the immune response is, and how well it holds up to future recurrences or metastases. If the results are confirmed, it may also be possible to use the vaccine in people at earlier stages of the disease, or even in people without melanoma who are at higher risk for it to prevent them from developing tumors in the first place.
KEYNOTE-942 is an ongoing randomized, open-label Phase 2b trial that enrolled 157 patients with stage III/IV melanoma. Following complete surgical resection, patients were randomized to receive mRNA-4157/V940 (nine total doses of mRNA-4157) and KEYTRUDA (200 mg every three weeks up to 18 cycles [for approximately one year]) versus KEYTRUDA alone for approximately one year until disease recurrence or unacceptable toxicity. The primary endpoint is recurrence-free survival, and secondary endpoints include distant metastasis-free survival and safety.
Key eligibility criteria for the trial included:
patients with resectable cutaneous melanoma metastatic to a lymph node and at high risk of recurrence, patients with complete resection within 13 weeks prior to the first dose of KEYTRUDA, patients were disease free at study entry (after surgery) with no loco-regional relapse or distant metastasis and no clinical evidence of brain metastases, patients had a formalin fixed paraffin embedded (FFPE) tumor sample available suitable for sequencing, Eastern Cooperative Oncology Group (ECOG) Performance Status 0 or 1 and patients with normal organ and marrow function reported at screening.
Companies plan to discuss results with regulatory authorities and initiate a Phase 3 study in melanoma in 2023 and rapidly expand to additional tumor types."
End ID.
"KEYTRUDA is an immunotherapy that works by increasing the ability of the body's immune system to help detect and fight tumor cells. Based on early clinical studies, combining mRNA-4157/V940 with KEYTRUDA may potentially provide an additive benefit and enhance T cell-mediated destruction of tumor cells.
Patients were treated and monitored for at least two years. The company has only reported patient outcomes so far- not details of the vaccinated patients’ immune responses, such as their T-cell levels, which vaccines train to recognize and eliminate pathogenic cells.
That data is being collected and that analysis will be provided in future presentations or publications. While there much more work to do, and scientists will attempt to figure out if the 44% reduction can be pushed even further, it is a transformational moment for the field of cancer treatment. No one has ever demonstrated that mRNA vaccines could work in a randomized controlled trial in cancer, but now, data is starting to show that they absolutely can. You can read more here:
https://time.com/6240538/mrna-cancer-vaccine-moderna/
https://investors.modernatx.com/news/news-details/2022/Moderna-and-Merck-Announce-mRNA-4157V940-an-Investigational-Personalized-mRNA-Cancer-Vaccine-in-Combination-with-KEYTRUDAR-pembrolizumab-Met-Primary-Efficacy-Endpoint-in-Phase-2b-KEYNOTE-942-Trial/default.aspx
23 notes
·
View notes
Text
The Best News of Last Week - May 16, 2022
🐶— Meet the good boi that has won hearts and admirers for detecting more than 200 undetonated explosive devices since the beginning of the war in late February
1. Spain set to become the first European country to introduce a 3-day ‘menstrual leave’ for women

Spain is set to offer three days of menstrual leave to women with severe period pain. The proposed policy will be voted on at a cabinet meeting next week, along with a measure to offer sanitary pads in schools.
“If someone has an illness with such symptoms, a temporary disability is granted, so the same should happen with menstruation ― allowing a woman with a very painful period to stay at home,” Angela Rodriguez, Spain’s secretary of state equality and gender violence, told El Periodico.
2. Leader of Pussy Riot Band Escapes Russia, With Help From Friends

Maria V. Alyokhina disguised herself as a food courier to evade the Moscow police who had been staking out the friend’s apartment where she was staying. She left her cellphone behind as a decoy and to avoid being tracked.
A friend drove her to the border with Belarus, and it took her a week to cross into Lithuania.
3. Patron the bomb-sniffing dog gets a medal from Zelenskyy

Patron poses at an award ceremony in Kyiv, Ukraine on Sunday. The Jack Russell terrier is credited with detecting more than 200 Russian explosive devices since the start of the war.
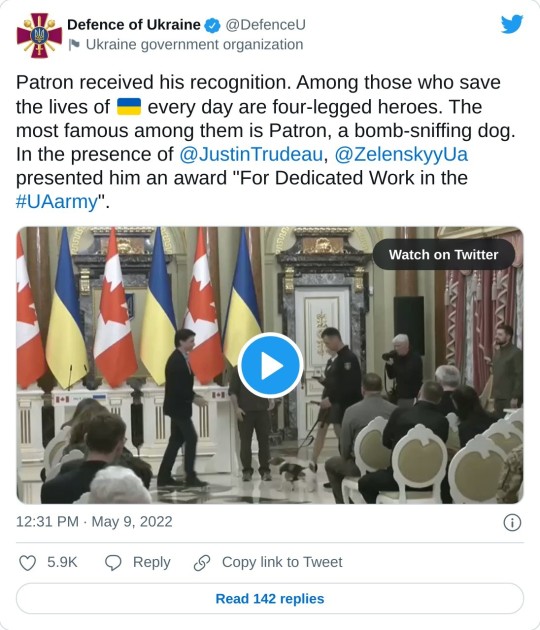
If you liked this post you can support this newsletter with a small kofi donation ❤️
4. Wolf seen in Brittany (France) for first time in a century

A single animal was filmed by a camera trap in the commune of Berrien, situated in the Arrée Mountains, on 3 May. Wolf was previously extirpated from the region in the early 20th century due to hunting pressures, and was later lost from the entirety of France.
5. Nonspeaking student with autism gives moving commencement speech
Rollins College’s Elizabeth Bonker who has not spoken since the age of 15 months due to autism delivered a moving commencement speech, urging her fellow graduates to use their own voices.
youtube
6. ‘Young stem cell’ transplant trial shows 5th ever case of human retinal tissue regeneration, with signs of vision improvement in macular degeneration — the leading cause of untreatable, aging-related blindness

New data from the phase 1 clinical trial examining OpRegen in patients with dry age-related macular degeneration (AMD) show that the subretinal cell therapy may help improve or maintain visual acuity in this patient population.
OpRegen has been generally well-tolerated with no unexpected adverse events.
7. Biggest ‘floating solar park’ in Europe will open this year in Portugal

Two tugboats are currently moving a vast array of 12,000 solar panels, the size of four football pitches, to their mooring on the reservoir. Miguel Patena, EDP group director in charge of the solar project, said on Thursday that electricity produced from the floating park, with installed capacity of 5 megawatts (MW), would cost a third of that produced from a gas-fired plant.
The solar panels will supply 1,500 families with power.
. . .
That's it for this week. Until next week, You can follow me on twitter. Also, I have a newsletter :)
Subscribe here to receive a collection of wholesome news every week in your inbox :D
234 notes
·
View notes
