#Transesterification
Photo
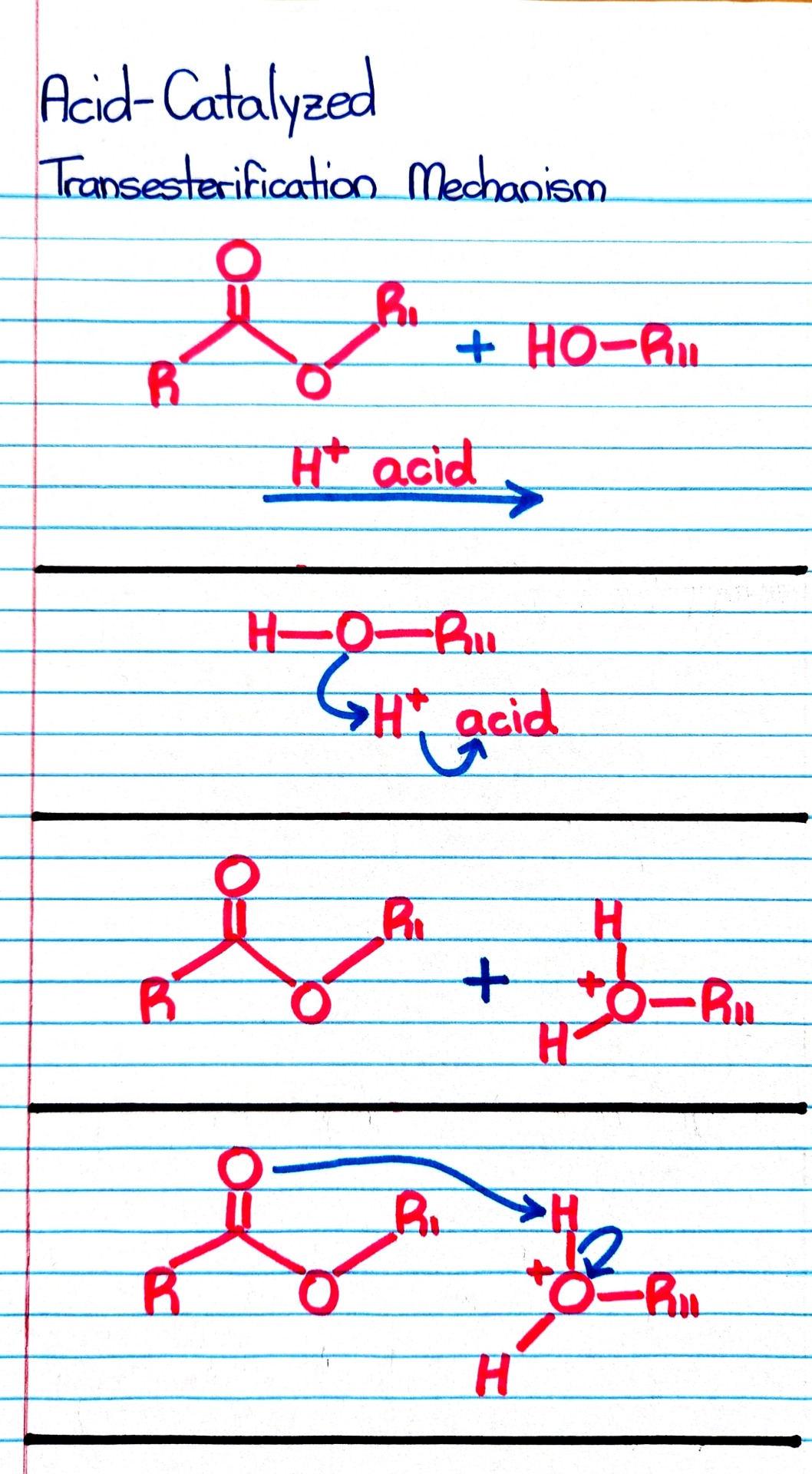
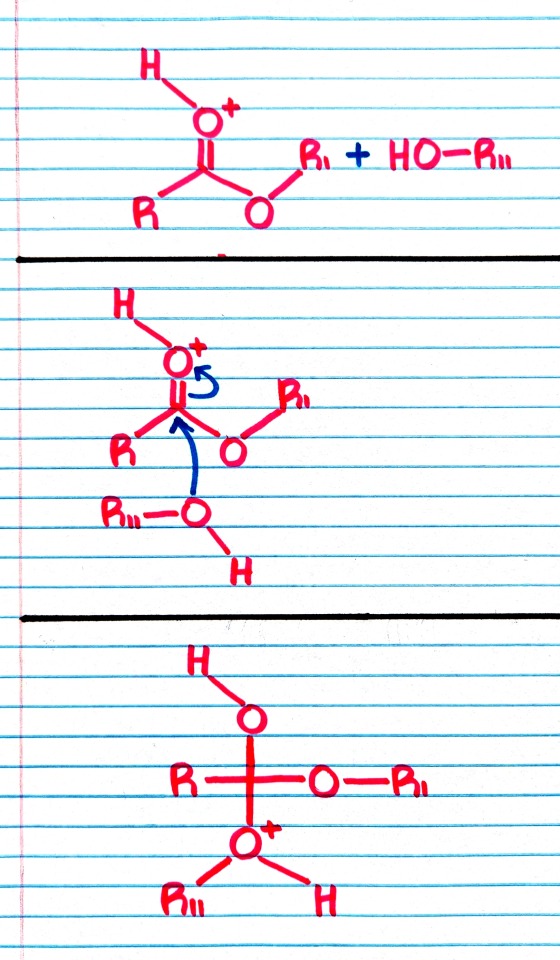
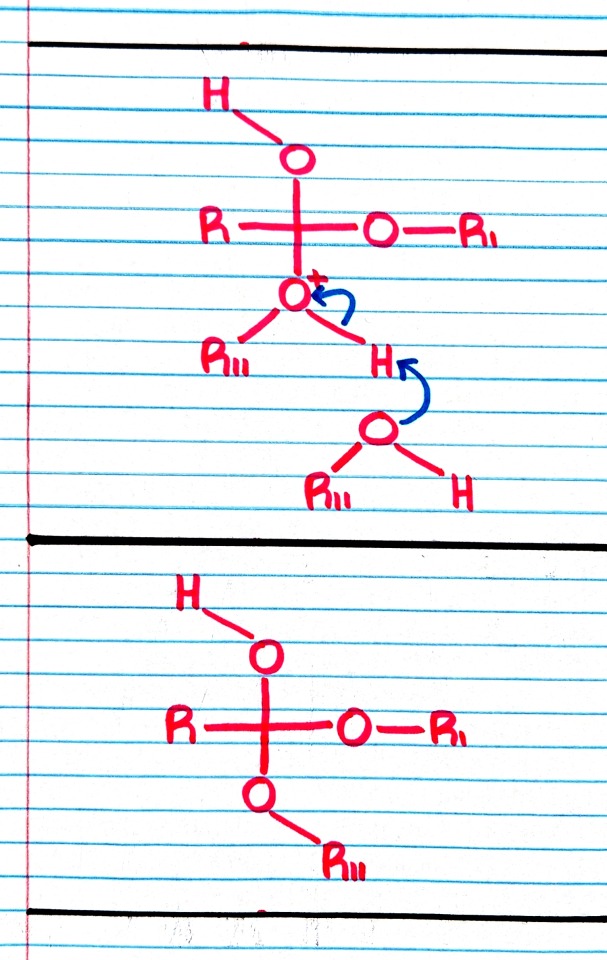
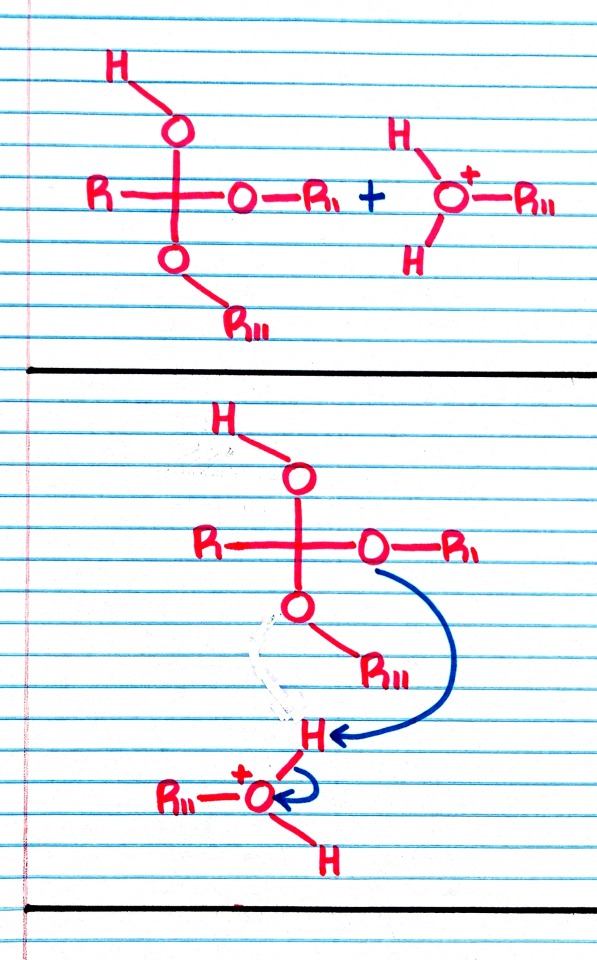
#organic chemistry#ochem#o-chem#o chem#ochem 1#ochem 2#organic chemistry 1#organic chemistry 2#mcat organic chemistry#orgo#reactions#orgo reactions#chemical reactions#orgo mcat#mcat orgo#ochem reactions#studyblr#notes#transesterification#esterification#transesterification notes#esterification notes#acid catalysis#acids#catalysis
2 notes
·
View notes
Text
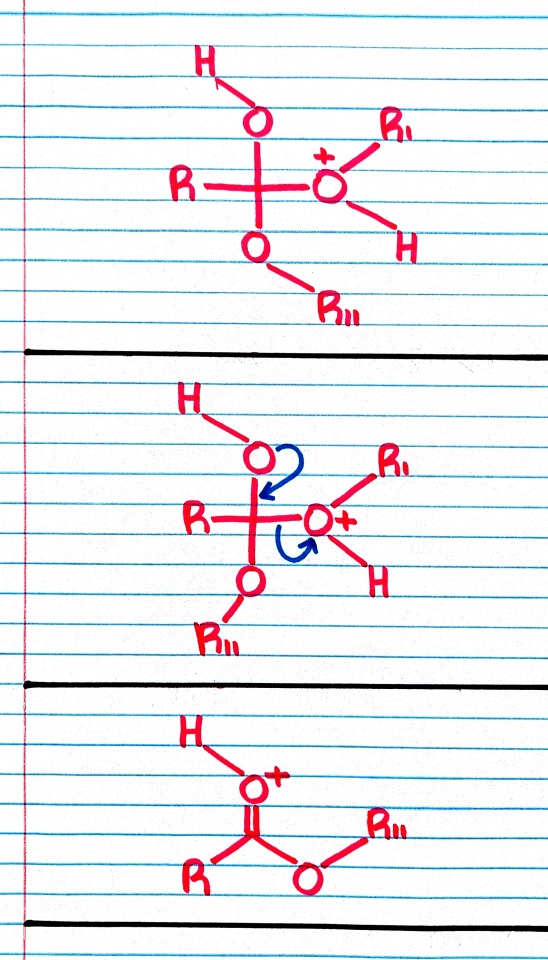
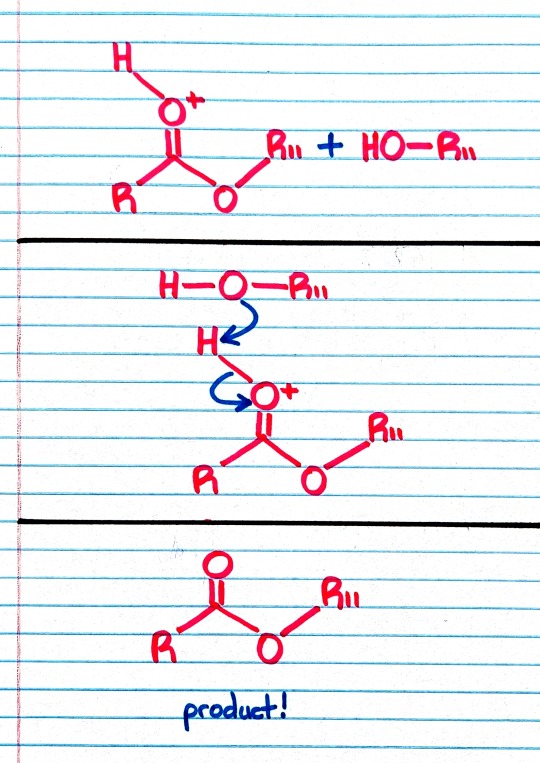
Treatment of an ester with an alcohol in the presence of an acid catalyst results in transesterification; that is, the replacement of one -OR group by a different -OR group.
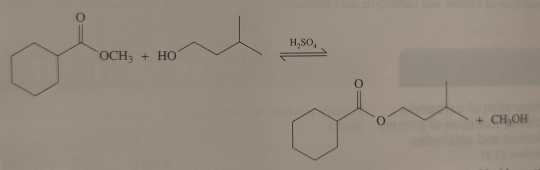
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#chemical reactions#ester#alcohol#acid#catalyst#transesterification#replacement
0 notes
Text
Caustic Soda
MCT Caustic Soda Prills (Sodium Hydroxide), weighing 17 kg, is readily available for shipping at a discounted price of AED 93.45 per piece, inclusive of VAT, with the option for easy installment payments. The product, composed of solid small fused white pearls that are color- and odorless, serves various purposes such as unblocking drains and soap making. Free of anti-caking and flow agents, it outshines conventional granules or flakes and finds significant applications in the paper and pulp industry. Moreover, it is a crucial component in soap, cleaning products, drain treatments, degreasing agents, oven cleaners, and is employed as an esterification and transesterification reagent in addition to its role in food preparation. The stock is located in the United Arab Emirates, with an estimated lead time of 4 business days, and international delivery options are available, along with associated costs and shipping times displayed during checkout.
Caustic Soda prills, also referred to as Sodium Hydroxide, is characterized by the chemical formula NaOH.
This inorganic compound serves a variety of purposes, including unblocking drains and soap production.
One of its key functions involves converting fats and grease that tend to clog pipes into soap through a chemical process.

#alramiz#are#MCT#CausticSodaPrills#SodiumHydroxide#ChemicalCompound#CleaningProducts#DrainTreatment#SoapMaking#DegreasingAgents#OvenCleaners#Esterification#Transesterification#PaperIndustry#PulpIndustry#ChemicalFormula#UnblockingDrains#SolidPrills#WhitePearls#VATInclusive#DiscountedPrice#InstallmentPayments#FreeOfAntiCakingAgents#InternationalDelivery#LeadTime#chemicalprocesses
1 note
·
View note
Text
What Is Transesterification
All the samples had been ready in batch reactors at the circumstances proposed within the design of experiments. In order to circumvent the leaching of the guanidines from the polymers, Schuchardt et al. encapsulate TCG by the response of dicyclohexylcarbodiimide and cyclohexylamine in the supercages of a hydrophobic Y zeolite. The encapsulated guanidine confirmed a great catalytic activity within the addition reaction of benzaldehyde to acetone71. However, its exercise within the transesterification of vegetable oils is low (14% conversion after 5 h72), because the diffusion of the triglycerides by way of the channels of the Y zeolite is gradual due to steric hindrance.
diesel generator installation
The physiochemical properties assessed includes viscosity, specific gravity, flash point, fire level, calorific value, cloud level, pour level, and carbon residue and ash content material. The results revealed a very significant distinction between the properties of bio-diesel and petroleum diesel. Although the enzyme-catalyzed transesterification processes are not yet commercially developed, new results have been reported in recent articles and patents42-48. The common features of those research consist in optimizing the reaction conditions to find a way to establish appropriate traits for an industrial utility. However, the reaction yields as well as the response instances are still unfavorable compared to the base-catalyzed response systems. The mechanism of the acid-catalyzed transesterification of vegetable oils is proven in Scheme 5, for a monoglyceride.
Then, the investigation outcomes using Paphiaundalata shell waste as heteregenous catalyst shows larger TE composition compared than homogenous catalyst . The formatted Jatropha biolubricant utilizing NaOH as catalyst at 200C reaches forty seven % TE maximal . The rised Otherwise, the elevated temperature greater than 110C displays the decreasing of TE, it might leads to vaporization of the reactant unstable substance. These experiments approves the obtained biolubricant with the higher TE as main product than the ME and DE asintermediate outcomes.
Wahlen method shows certain deficiencies when dealing with the fungal biomass, indicating a big matrix impact probably caused by the presence of polyphosphates and polysaccharides in the fungal cells. The significant difference in lipid yields outcomes, obtained by optimised and normal Lewis methods, indicates that some of the beforehand reported lipid yields should be corrected upwards. This could have necessary biotechnological implications for manufacturing of high-value (PUFA-rich) oils, as well as biodiesel, since it will indicate that some fermentation processes are extra economically viable than beforehand estimated. Finally, the research demonstrates value of biomass monitoring by FTIR, importance of optimal solvent to co-solvent ratio, as nicely as cautious choice and implementation of internal requirements for gas chromatography. However, the presence of high concentration of polyphosphates in fungal biomass, particularly in Mucor circinelloides, most likely hinders the transesterification means of TAGs because of the competing acid-based hydrolysis of polyphosphates.
Using the opposite bases, beneath the same experimental conditions, the yields weren't greater than 66% . The order of the catalytic activity is not directly related to the relative basicity of those compounds, since BEMP and Me7P must be the more environment friendly catalysts, adopted by TBD. However, the guanidines are more lively catalysts and the activity follows their relative basicity. The mechanism of the base-catalyzed transesterification of vegetable oils is proven in Scheme 6.
#biodiesel generator#diesel generator#biodiesel#fuels#diesel generator installation#transesterification
1 note
·
View note
Photo
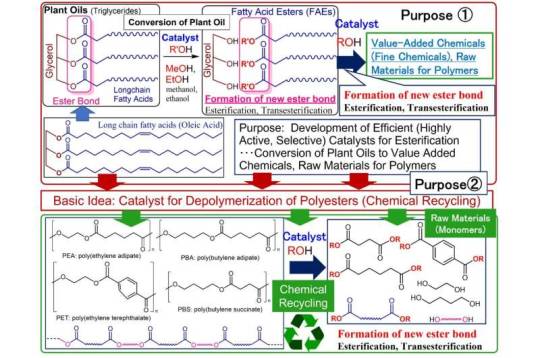
A high-performance catalyst that dissolves polyester and realizes chemical recycling
Professor Kotohiro Nomura's research group at Tokyo Metropolitan University developed two high-performance catalysts for efficient synthesis of value-added chemicals (fine chemicals, monomers) from polyester and vegetable oil. Their major finding is that simply heating a mixture of polyester and alcohol could convert the solution into raw materials. Their research is published in ACS Sustainable Chemistry & Engineering.
Plastic waste is an enormous environmental problem that needs to be solved immediately, but the amount of plastic being reused is still low. Polyesters, which consist of repeated "ester bonds" formed by the reaction of carboxylic acid and alcohol, are used in plastic bottles and clothing. If these ester bonds could be completely severed, polyester could be converted back into its raw materials. However, conventional methods require high temperatures and large amounts of acidic and/or basic materials. Therefore, a simple, inexpensive, and environmentally friendly method is desired.
Nomura's research group developed catalysts to facilitate the synthesis of high value-added chemicals (i.e., fine chemicals) such as the raw materials for polymers, detergents, and cosmetics from inedible vegetable oils, and discovered two types of high-performance catalysts: a calcium oxide catalyst and a titanium catalyst. These catalysts are effective for breaking down polyester based on the same chemical reaction (transesterification) and have been shown to be capable of converting polyester into raw materials with nearly 100% selectivity.
Read more.
46 notes
·
View notes
Text
PBT Unraveled: Exploring Applications and Manufacturing Methods

Polybutylene terephthalate, or PBT for short, might sound like a complex scientific mouthful. But behind this technical term lies a remarkable material with a wide range of applications in our everyday world. Chemically similar to its more well-known cousin PET (used in plastic bottles), PBT boasts impressive strength and crystalline structure. Today, we'll delve into the world of PBT, exploring its properties and the surprising places you might encounter this versatile material.
Introduction
Polybutylene terephthalate (PBT), a distinguished member of the polyester polymer family, has sparked considerable commercial interest owing to its remarkable versatility across a broad spectrum of applications. From automotive components to electrical and electronic devices, medical equipment, and beyond, PBT finds its place in an array of industries. Its extensive product range encompasses various grades tailored for injection molding, including reinforced, filled, impact-modified, and flame-retardant formulations. The unfilled PBT grades boast a diverse range of melt viscosities, offering ample processing flexibility in techniques like injection molding and extrusion. These techniques facilitate diverse applications, from crafting PBT fibers through melt-blowing to producing rods, slabs, fiber optic buffer tubes, and brake cable liners. Moreover, flame-retardant and lubricated versions of PBT are available in both filled and unfilled variants. Notably, glass-reinforced PBT grades exhibit enhanced mechanical properties compared to non-reinforced resins, showcasing significant increases in tensile, moduli, flexural, and compressive strengths, making them pivotal across numerous demanding applications.
PBT’s unique properties include:
Built to Last: PBT stands out for its dimensional stability and resistance to moisture. This makes it highly durable under heat and harsh chemicals.
Strength You Can Count On: Don't be fooled by its weight - PBT packs a punch. It boasts high strength, toughness, and stiffness, making it resistant to impact and deformation even at elevated temperatures.
Heat Doesn't Faze It: Bring on the heat! PBT has a high heat deflection temperature, meaning it can withstand both short bursts and long-term exposure to high temperatures without warping.
Electrical Champion: Looking for an insulator? Look no further! PBT offers excellent electrical resistance and dielectric strength, safeguarding electrical components from discharge and ensuring safe operation. The low dielectric loss makes it ideal for high-frequency electronics.
Chemical Fortress: Harsh chemicals are no match for PBT. It boasts resistance to a wide range of chemicals, from acids and solvents to oils and greases. Plus, it offers good UV and stain resistance, keeping things looking sharp.
Manufacturing Process
PBT is manufactured by polycondensation of terephthalic acid or dimethyl terephthalate with 1,4–butanediol using catalyst. The primary raw materials utilized in the production of PBT include 1,4-butanediol (BDO), dimethyl terephthalate (DMT), purified terephthalic acid (PTA), and catalysts.
In the PBT production process, a mixture comprising dimethyl terephthalate as the primary component of terephthalic acid alkyl ester and 1,4-butanediol (referred to as BD) as the main constituent glycol is blended in appropriate proportions within a mixing vessel.
A transesterification catalyst is introduced and conditioned in the mixture, which is then transferred to a transesterification reaction vessel via a pump, set to a predetermined reaction temperature.
During the transesterification reaction, two or three sequentially arranged stirring vessels equipped with stirring blades facilitate the process, leading to the formation of methanol as a by-product. Tetrahydrofuran (THF), resulting from the breakdown of methanol and also present with BD and water, is separated in a distillation tower. Subsequently, a polymerization catalyst is introduced, initiating the polymerization reaction stage. Initially, multiple vertical or horizontal stirring vessels are utilized for the prepolymerization phase, followed by a final polymerization step employing a horizontal stirring vessel.
In a continuous polycondensation process for materials like polyethylene terephthalate, operating within a relatively low viscosity range and under subatmospheric pressure. Oligomers with low polymerization degrees, resulting from esterification or transesterification reactions, are continuously supplied to one end of these reactors, allowing for the successive progression of the polycondensation reaction down to the downstream tray, or during the transfer of oligomers from one stirring vessel to another.
An apparatus designed for the continuous production of polybutylene terephthalate consists of a series of reactors: the first reactor facilitates the reaction between an aromatic dicarboxylic acid, primarily terephthalic acid or its derivative, and a glycol, mainly 1,4-butanediol. Subsequently, the oligomer undergoes polycondensation in the second reactor, generating a low polymerization product with an average degree of polymerization varying from 25 to 40. The third reactor further polycondenses the low polymerization product. Optionally, a fourth reactor is incorporated to further polycondense the polyester from the third reactor, achieving an average degree of polymerization of 150 to 200, thereby yielding a high molecular weight polyester characterized by superior heat stability and exceptional hydrolysis resistance. The first and second reactors operate without stirrers powered by an external source.
Within this apparatus, the second reactor takes the form of an approximately cylindrical vessel type, functioning as a flow reactor within a double cylinder structure. This reactor features an inner cylinder opening within the vessel and an inlet for the process solution at the lower part of the double cylinder structure. The process solution traverses through tubes of a shell and tube type heat exchanger positioned on the exterior of the inner cylinder, where it is heated to a predetermined temperature, then ascends to the level of the inner cylinder opening before descending through the inner cylinder. Throughout this process, the solution is stirred using a series of doughnut-type trays affixed to the inner wall of the outer cylinder. Additionally, the vessel includes an outlet for volatile matters and reaction by-products situated at its upper part. The inventors have identified areas for enhancement concerning the short pass and thermal decomposition reactions of the process solution.
Key PBT Technologies & Processes
Key licensors of PBT technology, such as Hitachi, Uhde Inventa-Fischer, and Lurgi Zimmer AG, offer a comprehensive range of process variations, covering both the DMT and PTA routes as well as batch and continuous processes. Various design configurations exist, ranging from a 5-Reactor (5-R) setup to a more compact 2-Reactor (2-R) arrangement. Recent advancements in technology, such as the Zimmer COMBI reactor, Uhde Inventa-Fischer ESPREE, and DISCAGE reactors, propose a 2-R design that integrates esterification and pre-polycondensation into a single reactor, thereby reducing overall investment costs.
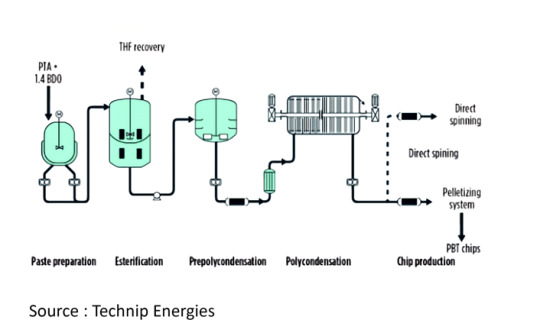
Zimmer Poly Butylene Terephthalate (PBT) Process
The primary starting materials for producing PBT are purified terephthalic acid (PTA) and 1,4-butanediol (BDO). A standard manufacturing facility is comprised of several key sections: raw material handling, paste preparation, esterification, prepolycondensation, final polycondensation, and pelletizing.
BDO, combined with a catalyst, is consistently mixed and introduced into the paste preparation vessel alongside terephthalic acid and comonomers at specific molar ratios. During the subsequent esterification phase, PTA and BDO react to create the ester bis-hydroxybutylterephthalate (BHBT) and oligomers, while also releasing water/THF.
The resultant product progresses to prepolycondensation, where the reaction continues, then moves on to the polycondensation stage. Here, the reaction concludes by removing BDO, residual THF, and water.
The polymer melt is then transferred via polymer discharge pumps, possibly passing through a polymer filter, for chip production. In this phase, the polymer is granulated using an underwater pelletizer and cooling system, preparing it for subsequent drying and packaging.
Vapors generated during esterification are released and channeled into a process column for rectification. BDO, BHBT, and oligomers are recycled back into the esterification process, while water/THF is directed for THF recovery.
The byproduct THF, predominantly produced during esterification, can undergo purification for further utilization in an additional rectification unit (a 3-stage column system).
Applications of Polybutylene Terephthalate (PBT)
Automotive
PBT is a versatile material used throughout cars, from external parts like windshield wiper covers and mirror housings to internal components like handles and fans. It's especially common in electrical systems, where it makes connectors, sensor housings, fuse boxes, and even parts of motors and ignition systems.
Electronics & Electricals
Because PBT is an excellent insulator, it stops electricity from leaking or causing breakdowns in electronic devices. This is why many different electrical parts are made from PBT. Here are some examples: switches, circuit breakers, power sockets, cable linings, connectors, and transformer insulation.
Industrial
PBT is tough stuff! It's strong, stiff, and can handle a good amount of impact, even in the short term. This makes it ideal for industrial parts that need to last a long time and take a beating. Think about things like fluorescent lamp bases, street lamp reflectors, pump parts, filters, and even packaging components. They all benefit from PBT's ruggedness.
Consumer Goods
PBT's strength and ability to handle heat and electricity make it a great choice for many everyday items. You'll find it in things like iron handles, oven door knobs, appliance housings, and even office furniture because it's both durable and looks good.
Market Outlook:
The demand for Polybutylene Terephthalate (PBT) is strongly influenced by the spanning Electrical & Electronics and Automotive industries. In Electrical & Electronics, PBT is favored for its excellent electrical properties at high temperatures, making it a preferred material for various components. Its high heat resistance and dimensional stability are particularly valued in this sector. Similarly, the Automotive industry plays a significant role in the PBT market, extensively using the thermoplastic in both interior and exterior automotive parts. PBT's ability to withstand high processing temperatures ensures the durability and performance of these parts. The demand from these sectors highlights PBT's versatility and reliability in meeting the stringent requirements of Electrical & Electronics and Automotive applications, positioning it as a key driver in the market.
Polybutylene Terephthalate (PBT) Major Players
Major players in the Global Polybutylene Terephthalate (PBT) market are Kanghui New Material Technology Co., Ltd., BASF, Henan Kaixiang Chemical, Lanxess/DuPont, Yizheng Chemical Fibre, Sabic Innovative Plastics, Nantong Xingchen Synthetic Material (Blue Star group), Saudi International Petrochemical (Sipchem), Toray BASF PBT Resin Sdn Bhd, Toray Japan, DuBay Polymer, Toray Industries (India) Private Limited, and Others.
Conclusion:
In conclusion, Polybutylene Terephthalate (PBT) is a formidable engineering plastic renowned for its robustness, rigidity, and superior machining attributes. With exceptional chemical resistance, along with outstanding bearing and wear properties, PBT stands as a premier choice in various industrial applications. PBT's properties such as electrical resistance and high dielectric strength make it useful in the electrical and electronic sectors. Also, the blend of excellent mechanical and electrical properties, along with strong thermal stability and chemical resistance, is the reason behind the usage of PBT in the automotive sector.
#PBT#PBTprices#PBTmarket#PBTpricetrend#PBTpriceforecast#PBTnews#PBTdemand#PBTsupply#PBTmarketprice#priceofPBT
1 note
·
View note
Text
Chemical Composition: Unveiling the Secrets of Methyl Myristate

Introduction:
Methyl Myristate, a compound with intriguing chemical composition, holds numerous secrets waiting to be uncovered.
Understanding its molecular structure and properties provides insights into its diverse applications across various industries.
Molecular Formula and Structure of Methyl Myristate
Methyl Myristate is an organic compound with the molecular formula C15H30O2.
Structurally, it consists of a 14-carbon fatty acid chain (myristate) attached to a methyl group.
This ester compound is synthesized through the reaction between myristic acid and methanol, resulting in its unique composition.
Hydrophobic Nature and Solubility
Methyl Myristate exhibits a hydrophobic nature due to its long hydrocarbon chain.
This hydrophobicity renders it insoluble in water but highly soluble in organic solvents such as ethanol, ether, and chloroform.
The solubility characteristics of Methyl Myristate influence its applications in various formulations and processes.
Fatty Acid Ester: Properties and Behavior
As a fatty acid ester, Methyl Myristate possesses distinctive properties and behavior.
It demonstrates good thermal stability, with a relatively low melting point and high boiling point.
These properties make Methyl Myristate suitable for use in applications requiring heat resistance and stability.
Synthesis Methods and Production Processes
Methyl Myristate is primarily synthesized through esterification reactions involving myristic acid and methanol.
The process typically occurs under acidic conditions and requires catalysts to facilitate the formation of the ester bond.
Alternative methods, such as transesterification, may also be employed for the production of Methyl Myristate from renewable feedstocks.
Get More Insights On This Topic: Methyl Myristate
#Methyl Myristate#Chemical Composition#Organic Compound#Cosmetics#Skincare#Fragrance Formulations#Industrial Applications#Emerging Uses
0 notes
Text
Exploring the Versatility of Polycarbonate: Applications, Manufacturing, and Advantages of Polycarbonate (2023-2034)

Polycarbonate is a common polymer which finds applications from automotive to consumer goods. In this blog, we'll dive into the fascinating world of polycarbonates. We'll explore its unique properties, its manufacturing process, and its range of applications that make it popular in the world of polymers. The global Polycarbonate market is likely to flourish at a moderate CAGR of 4.32% by the year 2034.
Here are some of the main topics we'll cover in this blog:
The Properties: Get to know the details of polycarbonate's remarkable properties like toughness, clarity, and heat resistance.
Applications: Explore the applications of polycarbonate, from lifesaving medical devices to everyday consumer products.
Introduction
Polycarbonate (PC) stands out as a high-performance thermoplastic polymer. Its molecular structure comprises organic functional groups connected by carbonate groups (–O–(C=O)–O–), granting it a distinctive array of properties. Due to its remarkable compatibility with certain polymers, polycarbonate finds extensive use in blends like PC/ABS, PC/PET, and PC/PMMA. This versatile material is employed in various applications, including compact discs, safety helmets, bulletproof glass, car headlamp lenses, baby feeding bottles, roofing, glazing, and more. With its exceptional toughness, transparency, and thermoplastic nature, polycarbonate remains a sought-after choice across industries, offering a balance of strength, durability, and optical clarity. Its ability to withstand impact, coupled with its transparent quality, renders it indispensable in safety-critical applications like protective gear and automotive components. Moreover, its versatility allows for diverse applications, ranging from consumer products to architectural solutions. Its properties include:
Transparency: PC boasts high transparency, facilitating superior light transmission. It is commonly utilized in applications necessitating optical clarity, like eyeglass lenses and transparent protective shields.
Impact resistance: A standout characteristic of PC is its outstanding resistance to impact. It is virtually indestructible, making it ideal for safety-critical applications such as bulletproof glass, safety goggles, and automotive headlight lenses.
Heat resistance: PC can endure high temperatures without melting or distorting. Its elevated glass transition temperature renders it suitable for applications requiring exposure to heat, such as microwave-safe cookware and LED light covers.
Lightweight: Despite its strength, PC is relatively lightweight. This property makes it favored for weight reduction in industries such as aerospace and automotive.
UV resistance: PC exhibits good resistance to ultraviolet (UV) radiation, making it suitable for applications like greenhouse panels or protective covers for outdoor equipment.
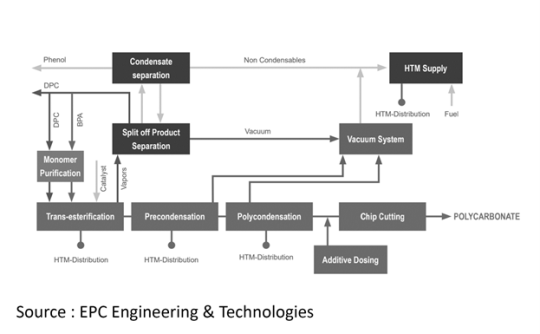
Manufacturing Process
VARIPLANT Process is the main process used to produce Polycarbonate. This process initiates Bisphenol A (BPA) and Diphenyl carbonate (DPC) melting which are then introduced into a raw material melt mixing tank. To ensure high-purity monomer essential for polycarbonate production, purification methods like filtration and impurity removal through stripping are applied. The pre-heated raw materials are then delivered in liquid form into the transesterification reactor. During the initial reaction phase, the raw material melt is combined with catalysts and heated to the specified transesterification temperature, adjusting a specific pre-conversion rate. As monomer and polymer chains develop, phenol begins to separate. Upon completion of transesterification, the produced oligomers are continuously discharged and directed to the prepolycondensation I reactor for the subsequent reaction phase.
By employing elevated temperatures and reduced vacuum conditions, mid-size chain length molecules are formed. Following the prepolycondensation I reactor (PP I), and depending on the plant's production rate design, the system can be configured with one, two, or three polycondensation lines in parallel. This allows for the simultaneous production of up to three distinct product grades. The product from PP I is then transferred to the prepolycondensation II reactor for further chain growth, and subsequently to the final polycondensation reactors. The final polycondensation reactor plays a crucial role in achieving the desired polymer chain length, thereby attaining the targeted properties of the Polycarbonate. Both the horizontal prepolycondensation II stage and the final reactor are equipped with a disc-ring agitator, ensuring significant surface area for efficient mass transfer and chemical reactions.
Applications of Polycarbonate
Electricals & Electronics
Polycarbonate and their blends find application in various appliances like refrigerators, air conditioners, coffee machines, and washing machines. Their utilization enables design flexibility due to a diverse range of mechanical properties, enhancing product durability and aesthetic appeal.
Automotive
Polycarbonate (PC), known for its lightweight and transparent nature, contributes to eye-catching vehicle designs and enhances efficiency by reducing weight without compromising durability or aerodynamics. Its exceptional heat resistance makes it ideal for light housing, headlamp bezels, and lenses. PC blends offer optimal rigidity and excellent creep resistance, making them suitable for both interior and exterior car body parts.
Construction
PC serves as a viable substitute for glass in diverse glazing applications including agricultural structures, industrial and public buildings, facades, security windows, shelters, and skylights. Its attributes of high impact strength, transparency, UV light resistance, and weatherability make it an ideal choice for such purposes.
Consumer goods
Polycarbonate's minimal birefringence, low internal stress, and precise dimensional accuracy make it ideal for CD/DVD manufacturing. Its exceptional transparency enables innovative designs for everyday items like safety goggles, ophthalmic lenses, and large-volume water bottles. Additionally, its optical clarity lends itself to applications such as shatterproof sunglasses, face shields, and bulletproof windows.
Market Outlook:
The automotive sector serves as the primary driver for the global Polycarbonate market, with its utilization in lightweight exterior and interior parts. Polycarbonate's unique properties enable sleek designs while reducing component weight by up to half, particularly in applications like automotive glazing, panoramic roof panels, and backlights. Additionally, its high impact resistance extends its usage to electronic devices, meeting the demand for durable and technologically advanced gadgets. The growing consumer preference for stylish automotive designs and innovative electronics further propels the demand for Polycarbonate, positioning it as a pivotal material in driving industry innovation and meeting evolving market demands. The global Polycarbonate market is anticipated to reach approximately 8.5 million tonnes by 2034.
Polycarbonate Major Manufacturers
Significant companies in the Global Polycarbonate market are Covestro AG, SABIC, Mitsubishi Engineering-Plastics Corporation, Lotte Chemical Corporation, LG Chem, Formosa Chemicals & Fibre Corp., Teijin Limited, Chi Mei Corporation, Idemitsu Kosan Co. Ltd. (Japan), Zhongsha (Tianjin) Petrochemical, SABIC-Sinopc JV, SHELL-CNOOC, LG Dow polycarbonate, Lutianhua Zhonglan New Materials, Wanhua Chemical, and Others.
Polycarbonate market restraints
The Polycarbonate market faces several restraints as well. These are as follows:
Fluctuating Raw Material Prices: Polycarbonate production relies on raw materials like bisphenol A and phosgene, the prices of which are subject to market volatility. Fluctuations in raw material costs can affect the overall production costs and profit margins for Polycarbonate manufacturers.
Environmental Concerns: The production process of Polycarbonate involves the use of chemicals and solvents that can have environmental implications. Stringent environmental regulations aimed at reducing emissions and waste disposal pose challenges for Polycarbonate manufacturers in terms of compliance and operational costs.
Competitive Pressure: The Polycarbonate market faces competition from alternative materials such as acrylics, polyethylene terephthalate (PET), and polystyrene (PS). These materials may offer similar properties at lower costs, posing a threat to Polycarbonate's market share.
Conclusion:
The global Polycarbonate demand, closely tied to Electrical and Electronics, Automotive, and Construction industries, has experienced strong growth in the past few years. Polycarboante’s distinct characteristics, including lightweight nature, high resilience, and resistance to chemicals, suggest an anticipated expansion of the Polycarbonate market in the forecast period. With urbanization activities on the rise, increasing demand for modern electronic devices, and heightened vehicle sales projected, there is expected to be a surge in demand for Polycarbonate by the year2034.
0 notes
Text
Turning Waste into Resource: The Process of Recycling Used Cooking Oil
In today's world, where sustainability is becoming increasingly crucial, finding innovative ways to repurpose waste materials is key to preserving our environment. One such example is the recycling of used cooking oil, which can be transformed into biodiesel—a renewable energy source. At Ecoil, we are committed to leading the way in this endeavor, turning waste into a valuable resource through our used cooking oil collection and recycling process.

The Importance of Recycling Used Cooking Oil: Used cooking oil is a common byproduct of households, restaurants, and food processing facilities. Improper disposal of this oil can lead to environmental pollution, clogged sewage systems, and harm to wildlife. The consequences of dumping used cooking oil down the drain or into the environment are severe, as it can coat waterways, suffocate aquatic life, and contaminate soil. By recycling used cooking oil, we can mitigate these negative impacts and create a sustainable solution for managing this waste product.
The Recycling Process:
Collection: Our first step in the recycling process is the collection of used cooking oil from various sources, including restaurants, commercial kitchens, and residential areas. We provide convenient collection services, ensuring that the oil is safely transported to our recycling facility. Our fleet of vehicles is equipped with specialized containers to prevent spillage and contamination during transport.
Inspection and Filtration: Upon arrival at our facility, the used cooking oil undergoes a thorough inspection to remove any contaminants or solid particles. Filtration processes are employed to separate impurities from the oil, ensuring that only clean oil is used in the recycling process. Our state-of-the-art filtration equipment ensures that the oil meets the highest quality standards before proceeding to the next stage of the process.
Conversion to Biodiesel: Once the oil has been purified, it is ready for the transformation into biodiesel. Through a chemical process called transesterification, the used cooking oil is converted into biodiesel—a renewable fuel that can be used to power vehicles and machinery. During transesterification, the triglycerides present in the oil are reacted with alcohol (usually methanol or ethanol) and a catalyst (such as sodium hydroxide or potassium hydroxide) to produce biodiesel and glycerin. The glycerin is then separated from the biodiesel and can be used in various other applications, such as soap production. This biodiesel is eco-friendly, emitting fewer greenhouse gases and pollutants than traditional diesel fuel.
Quality Assurance: Before the biodiesel is distributed for use, it undergoes rigorous quality assurance testing to ensure that it meets industry standards and specifications. Our commitment to quality ensures that our biodiesel is of the highest caliber, delivering optimal performance and environmental benefits. We conduct tests for key parameters such as viscosity, flash point, acid value, and cetane number to ensure that the biodiesel meets all regulatory requirements.
The Environmental Impact: Recycling used cooking oil into biodiesel offers significant environmental benefits. By reducing the reliance on fossil fuels, biodiesel helps decrease greenhouse gas emissions and combat climate change. Additionally, the recycling process reduces waste and pollution, contributing to a cleaner and healthier planet for future generations. Biodiesel is biodegradable and non-toxic, making it safer for the environment and human health than conventional petroleum-based fuels. Furthermore, biodiesel has a lower carbon footprint compared to traditional diesel fuel, as it is produced from renewable resources such as vegetable oils and animal fats.
Conclusion: At Ecoil, we believe in the power of innovation and sustainability to create positive change in the world. Through our used cooking oil collection and recycling process, we are turning waste into a valuable resource, one biodiesel batch at a time. Join us in our mission to protect the environment and build a greener, more sustainable future for all. Together, we can make a difference and leave a legacy of environmental stewardship for generations to come.
0 notes
Text
Biodiesel Market Revenue to Cross USD 187.6 billion by 2031, Registering at a CAGR of 6.9% from 2021 to 2031
The biodiesel market is experiencing remarkable growth as the world shifts towards sustainable energy sources and seeks alternatives to fossil fuels. With advancements in technology, supportive regulatory frameworks, and growing environmental concerns, biodiesel presents a promising solution for reducing carbon emissions and achieving energy security.
The global biodiesel market stood at US$ 90.4 billion in 2020, and the global market is projected to reach US$ 187.6 billion by 2031. The global industry is anticipated to record a CAGR of 6.9% between 2021 and 2031.
The value of the biodiesel market is increasing, owing to increasing environmental concerns and climate change mitigation. There is a rising emphasis on reducing carbon footprints and transitioning to cleaner energy sources, with growing awareness of the detrimental effects of greenhouse gas emissions and the need to combat climate change.
Biodiesel, produced from renewable feedstocks such as vegetable oils, animal fats, and used cooking oil, offers a significant reduction in greenhouse gas emissions compared to conventional diesel fuel. The environmental advantage positions biodiesel as a viable solution for sustainable transportation and energy sectors.
Discover a detailed summary of the report, encompassing market size, forecast, and research methodology. Access the sample report in PDF format @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=291
Market Segmentation:
By Service Type: Production, Distribution, Retail
By Sourcing Type: Vegetable Oil, Animal Fat, Recycled Cooking Oil, Others
By Application: Transportation, Industrial, Agriculture, Power Generation, Others
By Industry Vertical: Automotive, Agriculture, Chemicals, Power Generation, Others
By Region: North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Regional Analysis:
North America: Leading the biodiesel market with a well-established infrastructure and supportive policies promoting biofuel production and consumption.
Europe: Strong regulatory framework promoting renewable energy sources and reducing carbon emissions, driving investments in biodiesel production and usage.
Asia Pacific: Witnessing rapid industrialization and urbanization, leading to increasing demand for biodiesel as a clean and sustainable fuel alternative.
Market Drivers and Challenges:
Drivers: Growing awareness of environmental sustainability, government incentives and mandates promoting biofuel use, volatility in crude oil prices, and technological advancements in biodiesel production processes.
Challenges: Fluctuating feedstock prices, competition from other renewable energy sources, infrastructure constraints for biodiesel distribution, regulatory uncertainties, and compliance requirements.
Market Trends:
Advanced Feedstock Conversion: Adoption of advanced technologies such as enzymatic transesterification and hydroprocessing for efficient conversion of feedstock into biodiesel, enhancing yield and quality.
Blending Mandates: Implementation of blending mandates requiring a certain percentage of biodiesel in conventional diesel fuel, driving demand for biodiesel and encouraging investment in production capacity.
Sustainable Sourcing: Emphasis on sustainable sourcing of feedstock to mitigate environmental impact and ensure social responsibility throughout the biodiesel supply chain.
Future Outlook: The biodiesel market is expected to witness significant growth in the coming years, driven by increasing environmental awareness, regulatory support, and technological innovations. Expansion of production capacity, diversification of feedstock sources, and investments in infrastructure will shape the future outlook of the biodiesel industry.
Key Market Study Points:
Analysis of biodiesel consumption trends across different sectors and regions, including transportation, agriculture, and industrial applications.
Assessment of government policies and incentives influencing biodiesel production, distribution, and consumption.
Technological advancements in biodiesel production processes, feedstock cultivation, and fuel blending techniques.
Market dynamics affecting feedstock prices, fuel prices, and competitive landscape.
Environmental and social impact assessment of biodiesel production and usage.
Competitive Landscape:
The biodiesel market is characterized by the presence of key players such as Archer Daniels Midland Company, Cargill, Inc., Renewable Energy Group, Inc., and Wilmar International Limited. These companies are actively engaged in expanding production capacity, diversifying feedstock sources, and investing in research and development to enhance product quality and sustainability.
Recent Developments:
Development of next-generation biodiesel technologies using algae and microbial fermentation for feedstock production.
Expansion of biodiesel production facilities and acquisition of new assets to meet growing market demand.
Collaboration with automotive manufacturers and fuel retailers to promote biodiesel usage and infrastructure development.
Investment in sustainability initiatives such as certification programs and carbon offset projects to enhance the environmental profile of biodiesel products.
About Transparency Market Research
Transparency Market Research, a global market research company registered in Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision-makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyze information.
Our data repository is continuously updated and revised by a team of research experts so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll-Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
0 notes
Text
Brazil Biofuels Market Will Grow At Highest Pace Owing To Increasing Demand For Cleaner Fuels

Brazil biofuels market involves production and usage of biofuels like ethanol and biodiesel. Ethanol in Brazil is primarily produced from sugarcane and its juice which is fermented to produce fuel-grade ethanol. Brazil is the second largest producer of ethanol globally and majority of the ethanol produced is used as a gasoline blend. Biodiesel in Brazil is primarily produced through transesterification of vegetable oils like soybean oil. Biofuels provide various advantages like lowering of greenhouse gas emissions, reduction in dependency on fossil fuels, and boost to the country's agricultural sector. The growing concern about climate change and need for sustainable fuels is driving the demand for biofuels in Brazil.
The Global Brazil Biofuels Market Size Is Estimated To Be Valued At US$ 8.22 Bn In 2024 And Is Expected To Exhibit A CAGR Of 9.6% Over The Forecast Period 2024-2031.
Key Takeaways
Key players operating in the Brazil biofuels market Are Raízen, Granbio, Bsbios, Ipiranga, Petrobras, Adm, Louis Dreyfus Company, Bunge, Cargill, And Biosev. Raízen is the largest bioethanol producer in Brazil. GranBio is pioneering the commercial scale production of cellulosic ethanol.
The demand for biofuels especially ethanol is growing in Brazil due to the blending mandate set by the government. As per the mandate, gasoline sold in the country must have at least 27% of anhydrous ethanol blended into it. This mandate ensures strong offtake of ethanol from sugarcane producers.
Advancements are being made in cellulosic ethanol and biodiesel production technologies which can help Brazil tap into wider feedstock sources and reduce production costs. Companies like GranBio are working on developing technologies for producing ethanol from agricultural residues and energy crops.
Get more insights on this topic: https://www.pressreleasebulletin.com/brazil-biofuels-market-trend-size-growth-and-demand/
#Brazil Biofuels Market Trend#Brazil Biofuels Market Growth#Brazil Biofuels Market Analysis#Brazil Biofuels Market Size
0 notes
Text
Cutting-edge technologies for Biodiesel Plant .
The biodiesel industry is rapidly evolving with the introduction of cutting-edge technologies that enhance efficiency, reduce environmental impact, and expand feedstock options. Here’s an overview of some of the latest advancements in bio diesel plant technology
High-Efficiency Reactors
The rate at which feed stock is converted to bio-diesel has increased dramatically in modern reactors, boosting plant productivity. The transesterification process, which is the chemical reaction that turns oils and fats into biodiesel, is optimized by the design of these reactors.
Innovative Catalysts
The creation of new catalysts, like solid and enzyme-based catalysts, has revolutionized many industries. Compared to conventional sodium or potassium hydroxide, these catalysts are more effective and environmentally benign, minimizing waste and improving the biodiesel manufacturing process.
Continuous Flow Systems
Systems for producing biodiesel with continuous flow are gaining popularity because they simplify operations and lower expenses. Continuous biodiesel production is made possible by these technologies, which can result in increased throughput and decreased downtime.
Feedstock Flexibility
Technological developments have made it possible for biodiesel facilities to utilize a greater variety of feedstocks, such as animal fats, algae, and leftover cooking oil. This adaptability encourages the use of waste materials and lessens competition for food-grade crops.
Control and Automation
Modern biodiesel plants that integrate advanced automation and control technology yield production that is more precise and dependable.
Energy Efficiency
Energy-efficient designs are at the forefront of new biodiesel plant constructions. These designs seek to increase the sustainability of the facilities and lessen the carbon impact of the production process.
The manufacturing of biodiesel is looking more promising and profitable thanks to these technological developments. We may anticipate other developments as the sector expands, which will enhance the procedure and raise the appeal of biodiesel as a fossil fuel substitute. Please ask questions if you need more particular details about any area of biodiesel technology!
to know more
please feel free to contact us 6289715123
visit our site - magellanium.com biodiesel plant manufacturer in india
#biodiesel#business#chemical industry#industrial#industry analysis#biodiesel plant manufacturer#biodiesel equipments
0 notes
Text
Aquatic Solutions: Addressing Global Challenges with Marine Biotechnology
Introduction to Marine Biotechnology
Marine biotechnology utilizes living marine organisms and their derivatives for industrial, medical, environmental and other sustainable applications. It focuses on exploring the immense biodiversity and biological resources found in the marine environment. The oceans cover over 70% of the Earth's surface and contain a vast variety of lifeforms that have evolved unique biological properties and molecules. Marine biotechnology aims to unlock this untapped potential through research and innovations.
Marine Bioprospecting
Marine bioprospecting involves searching oceans, coastal waters and marine life for bioactive compounds, genes and other materials that can be exploited commercially. Some key activities include monitoring biodiversity hotspots, collecting and screening biological samples for useful properties, isolating lead compounds and developing applications. Marine organisms like sponges, corals, microbes and algae have yielded molecules with diverse pharmaceutical applications. Compounds used in cancer treatments, anti-inflammatories and antimicrobials have been derived from marine sources through bioprospecting. Advances in genomics and metabolomics also aid the drug discovery process from marine natural products.
Biomedical Applications
Marine-derived pharmaceuticals represent a promising area within marine biotechnology. Several drugs developed from marine sources are already in clinical use or under clinical trials. compounds obtained from marine sponges have led to antiviral and anticancer drugs. Ziconotide, an analgesic developed from a cone snail peptide, is used for severe chronic pain. Ecteinascidin 743, an anticancer compound from a marine tunicate is marketed for treating soft tissue sarcomas and other cancers. Fucoidan from brown algae shows anti-inflammatory and anticoagulant properties. Research continues to explore more marine organisms for bioactives against diseases like arthritis and Alzheimer’s. Marine enzymes also offer opportunities in areas like tissue engineering and wound healing.
Aquaculture and Mariculture
Marine biotechnology helps improve aquaculture practices and marine farming techniques. Genetic improvements through selective breeding and biotechnology tools augment disease resistance in cultured species. Microalgae and feed supplements utilizing marine microbes aid larval and post-larval development. Bioflocs containing waste-digesting bacteria provide eco-friendly water treatment in recirculating aquaculture systems. Bioremediation using micro/macro algae assists effluent treatment from aquafarms. Marine bacterial extracts serve as immunostimulants and natural healthcare alternatives in shrimp and fish mariculture. Advances accelerate sustainable production and higher yield in mariculture to meet the global seafood demand.
Environmental Applications
Biotechnological methods help address various environmental issues affecting the oceans. Bioremediation leverages metabolically versatile marine microbes like algae, fungi and bacteria to detoxify pollutants and rehabilitate contaminated coastal and offshore sites. Phytoremediation utilizes salt-tolerant plants to remove heavy metals and nutrient runoff from seawater. Genetic engineering modifies oil-degrading bacteria to ensure faster oil spill cleanup. Biosensors incorporating marine enzymes and whole-cell detection systems enable real-time coastal pollution monitoring. Bioluminescent bacteria offer scope in marine biomonitoring as indicators of toxicity and contamination levels. Such green technologies aid responsible utilization of marine resources and their conservation.
Energy from Oceans
Marine biomass represents a renewable source of bioenergy. Micro/macroalgae can be converted to liquid biofuel through transesterification and fermentation. Seaweed cultivation coupled with pyrolysis or gasification produces biogas, while anaerobic digestion generates methane from marine biomass. Biotechnological research optimizes algal strains, development of efficient conversion processes and validation of techno-economic models to tap the ocean's energy potential viably. Microbial fuel cells leveraging exoelectrogenic marine bacteria directly convert biochemical energy to electricity. Osmotic power utilizes blue energy from salinity differences between seawater and rivers. Wave and tidal energies extracted through emerging marine hydrokinetic technologies add to the blue energy basket.
Challenges and Future Prospects
While offering immense promise, marine biotechnology market still faces challenges in areas like cost-effective production, stability of marine compounds, regulatory approvals and public acceptance of ocean-based GM technologies. Adverse impacts of climate change on marine ecosystems and dwindling natural resources also demand mitigation. Integrated efforts towards exploration of deep-sea resources, metagenomic studies on uncultured microbes, synthesis of unique marine biomolecules and developing marine-derived industrial bioprocesses can significantly advance the sector. Public-private partnerships, internationally coordinated research initiatives and responsible scientific stewardship hold the key to realizing marine biotech’s full potential sustainably in the times ahead.
In conclusion, with over 70% of our planet covered by oceans, marine biotechnology market presents a vital avenue to harness the vast treasure of marine biodiversity alongside alleviating challenges on land and seas. A blending of marine sciences with industrial biotechnology promises to deliver innovative solutions across medicine, aquaculture, bioremediation and renewable energy. Sustained efforts to develop advanced techniques, unlock marine genome secrets and scale up
0 notes
Text
Biofuel Lubricity Improvers Market Size, Share, Trends, Growth Opportunities and Competitive Outlook
Biofuel Lubricity Improvers Market report is an important manuscript for every market enthusiast, policymaker, investor, and market player. The market research and analysis conducted in this report assists clients in forecasting the investment in an emerging market, growth of market share or success of a new product. In addition, this business report endows with a delegate overview of the market where it identifies industry trends, determines brand awareness, potency and insights and provides competitive intelligence. Report contains strong and weak points of the competitors and analysis of their strategies with respect to product and industry. Biofuel Lubricity Improvers Market is the most established tool and hence used widely to generate market research report.
With the complete understanding of business environment that is best suitable for the requirements of the client, Biofuel Lubricity Improvers Market business report has been generated. Businesses can also achieve insights into profit growth and sustainability programs with this market report. Market drivers and market restraints explained in this report gives idea about the rise or fall in the consumer demand for the particular product depending on several factors. This market document contains all the company profiles of the major players and brands. Each of the topics is properly elaborated with the in-depth research and analysis for generating an absolute Biofuel Lubricity Improvers Market survey report.
Data Bridge Market Research analyses that the biofuel lubricity improvers market will witness a CAGR of 6.4% for the forecast period of 2022-2029 and is likely to reach at USD 1415.6 million by 2029.
Access Full 350 Pages PDF Report @
Biodiesel is an alternate fuel derived from the transesterification of vegetable oils or animal fats, possesses inherent lubricity. It's additionally been usually accepted that biodiesel will restore lubricity to low-sulfur diesel fuels at mix levels of 1-2% biodiesel in petrodiesel fuel.
Some of the major players operating in the biofuel lubricity improvers market report are Biofuels Digest, Neste Netherlands B.V., Infinita Renovables SA, Marseglia Group, Glencore, Louis Dreyfus Holding B.V., Renewable Energy Group, Inc., RB FUELS, Ag Processing, Inc., Elevance, Marathon Petroleum Corporation, Evergreen Bio Fuels, Minnesota Soybean Processors, Caramuru., ENF Ltd., Hebei Jingu, Hebei Jingu, Shandong Jinjiang, Valero Marketing and Supply Company, Green Plains Inc., Flint Hills Resources, Abengoa Bioenergy, Pacific Ethanol, Inc., CropEnergies AG, Raízen, The Andersons, Inc. and BTG International Ltd among other.
Biofuel Lubricity Improvers Key Benefits over Global Competitors:
The report provides a qualitative and quantitative analysis of the Biofuel Lubricity Improvers Market trends, forecasts, and market size to determine new opportunities.
Porter’s Five Forces analysis highlights the potency of buyers and suppliers to enable stakeholders to make strategic business decisions and determine the level of competition in the industry.
Top impacting factors & major investment pockets are highlighted in the research.
The major countries in each region are analyzed and their revenue contribution is mentioned.
The market player positioning segment provides an understanding of the current position of the market players active in the Personal Care Ingredients
Table of Contents: Biofuel Lubricity Improvers Market
1 Introduction
2 Global Biofuel Lubricity Improvers Market Segmentation
3 Executive Summary
4 Premium Insight
5 Market Overview
6 Biofuel Lubricity Improvers Market, by Product Type
7 Biofuel Lubricity Improvers Market, by Modality
8 Biofuel Lubricity Improvers Market, by Type
9 Biofuel Lubricity Improvers Market, by Mode
10 Biofuel Lubricity Improvers Market, by End User
12 Biofuel Lubricity Improvers Market, by Geography
12 Biofuel Lubricity Improvers Market, Company Landscape
13 Swot Analysis
14 Company Profiles
Critical Insights Related to the Biofuel Lubricity Improvers Included in the Report:
Exclusive graphics and Illustrative Porter’s Five Forces analysis of some of the leading companies in this market
Value chain analysis of prominent players in the market
Current trends influencing the dynamics of this market across various geographies
Recent mergers, acquisitions, collaborations, and partnerships
Revenue growth of this industry over the forecast period
Marketing strategy study and growth trends
Growth-driven factor analysis
Emerging recess segments and region-wise market
An empirical evaluation of the curve of this market
Ancient, Present, and Probable scope of the market from both prospect value and volume
Browse Trending Reports:
Starch Blends Biodegradable Plastic Market
Onshore Drilling Fluids Market
Ballistic Composites Market
Organic Peroxide Market
Phosphate Rock Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
0 notes
Text
Gleaming into Glycerine: Manufacturing Processes and Applications

Glycerin might seem like a basic ingredient, a clear, odorless liquid found on countless product labels. But behind its simple appearance lies a world of surprising benefits. From keeping your skin healthy to aiding medical treatments, Glycerin's uses extend far beyond the cosmetic aisle.
In this blog post, we'll delve into the fascinating world of Glycerin. We'll explore its surprising origins, from everyday sources like vegetable oils to its role in industrial applications. We'll also uncover the science behind Glycerin's effectiveness in skincare and its potential health benefits. So, whether you're a curious consumer or a skincare enthusiast, get ready to discover the hidden potential of this versatile molecule!
Introduction
Glycerin, a dense and transparent liquid devoid of scent, offers a multitude of advantages spanning from medicinal applications to cosmetics. Its versatility manifests in skincare routines, either in its pure form or as an ingredient integrated into various beauty products. Derived from sources like soybean, palm oil, coconut oil, as well as animal origins and petroleum, Glycerin finds extensive industrial utility. Its diverse applications encompass the manufacturing processes of explosives, paints, varnishes, inks, textiles, and adhesives.
Commonly recognized under the names glycerol and Glycerine, this compound falls under the category of sugar alcohols due to its chemical structure, although it lacks any intoxicating properties. It exhibits complete solubility in water, ether, and alcohol, alongside its hygroscopic nature, facilitating the absorption of moisture from the environment. Endorsed by the Food and Drug Administration (FDA) as a safe food additive, Glycerin holds the generally recognized as safe (GRAS) status and earns approval for incorporation into skincare formulations and other cosmetic products. Remarkably, it ranks as the third most prevalent ingredient in cosmetics.
Manufacturing Process
Glycerin can be derived from various industrial processes, including methylesters production, fats saponification, and fat splitting, each involving different by-products. Depending on the initial by-product, the production process varies. For by-products like sweet water from fat splitting and spent lyes from saponification, the process entails chemical treatment, concentration, Glycerin distillation, and refining.
In the treatment phase, hydrochloric acid is utilized to reduce the solubility of fatty acids, which are then separated. Sweet water undergoes a single filtration before distillation, while spent lyes are filtered twice, both before and after neutralization.
The crude Glycerin undergoes further processing in a distillation, deodorizing, and bleaching facility. The distillation process involves washing, rectification, and condensation steps.
In the final purification stage, activated carbon is used in the bleaching section to eliminate color and odor, resulting in pure Glycerin.
Transesterification
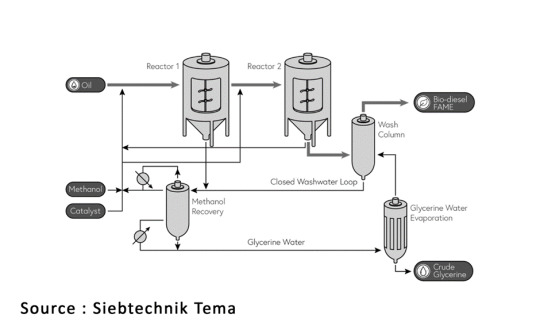
Transesterification process involves the conversion of methyl esters from triglycerides (oils) and methanol (alcohol) into glycerol and fatty esters (or biodiesel). Both homogeneous and heterogeneous catalysis methods are employed in the production of biodiesel and consequently, glycerol.
Initially, vegetable oils react with methanol in the presence of the catalyst. Subsequently, glycerol separation from the product mixture occurs via a settler unit. The residual flow undergoes treatment in a unit designed to remove the catalytic component using mineral acids, resulting in the generation of two streams: one for glycerol recovery and another for an evaporator, which separates biodiesel from other by-products. The glycerol purification unit yields three output streams: the first containing 80%–95% glycerol, the second comprising water, dissolved salts, and unreacted methanol (recycled back to the reactor), and the third stream containing fatty esters.
To enhance the conversion of vegetable oil, the process involves two reaction steps. In the first reactor, vegetable oil and methanol are introduced. The resulting product stream undergoes heat exchange to vaporize some unreacted methanol, with the remainder directed to a decanter for the separation of polar (predominantly glycerol) and non-polar (mostly vegetable oil and biodiesel) components. Subsequently, the non-polar stream undergoes a second reaction in the second reactor to further increase biodiesel production and recover methanol. Here, the product stream undergoes heat exchange to remove all unreacted methanol, while the decanter separates biodiesel from polar components. The polar streams from both decanters are directed to another heat exchanger to recover any remaining methanol, with the residual portion directed to a final decanter for the separation of vegetable oil and unreacted glycerol.
SIEBTECHNIK GMBH, one of the leading producers of Glycerin also uses this method. Bio-diesel production involves the transesterification of fats or oils with methanol, typically catalyzed by a basic catalyst. This reaction converts one mole of triglyceride into three moles of biodiesel (ester) and one mole of glycerol. Consequently, each batch of biodiesel generates approximately 10% by weight of glycerol. This glycerol, known as crude glycerol, is a by-product of the transesterification process. The transesterification reaction is pivotal in transforming renewable fats and oils into biodiesel, replacing glycerol molecules in triglycerides with methanol to yield biodiesel and glycerol. It's worth noting that the glycerol obtained in this process is impure, containing various contaminants, hence its classification as crude glycerol. Proper management of this by-product is crucial for bio-diesel production, ensuring efficient resource utilization and environmental sustainability.
Propylene Chlorination
In the propylene chlorination process, allyl chloride is generated at a temperature of 510°C in the presence of hypochlorous acid at 38°C. Subsequently, allyl chloride undergoes a reaction to form Glycerine dichlorohydrine. Following this, glycerol dichlorohydrine is hydrolyzed either by caustic soda in a 6% Na2CO3 solution at 96°C or directly into Glycerine, with epichlorohydrin removed as overhead in a stripping column. Finally, epichlorohydrin is hydrated to Glycerine using caustic soda. This process enables a final glycerol yield of approximately 90%.
Saponification
The traditional method of splitting natural fats and oils through saponification with alkali, such as caustic soda or sodium hydroxide, has been practiced for centuries. Commonly employed in this process are caustic alkali or alkali carbonates. Alternatively, calcium hydroxide in the form of milk of lime can also serve as a reagent. In this saponification procedure, fats and oils are heated with a caustic soda solution and salt. The triglycerides within the fats and oils react with the caustic soda, resulting in the formation of soap and glycerol. The addition of salt induces the separation of the mixture into two distinct layers – the upper layer consisting of soap and the lower layer, known as spent lye, containing glycerol, water, excess caustic soda, and salt. Continuous saponification processes, known as consap, are also utilized for soap production.
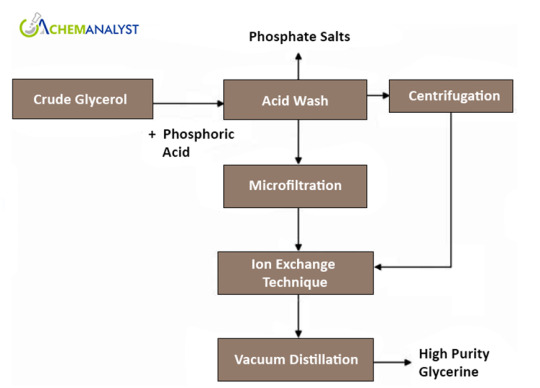
REFINING
Refining crude natural glycerol typically involves distillation, often followed by activated carbon treatment. In some instances, ion exchange is utilized.
Distillation
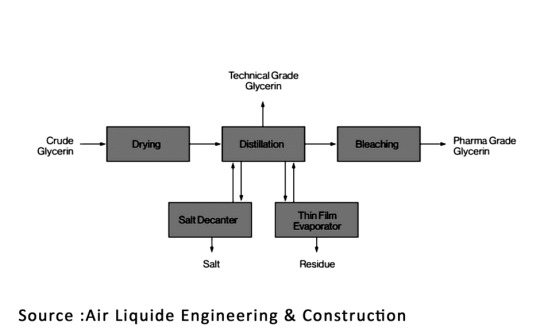
The process utilizes vacuum distillation to separate Glycerine from organic components and salts, operating at temperatures of up to 175°C. The remaining substance is then directed to a thin film evaporator to enhance Glycerine yield. Furthermore, a decanter is employed to separate salt from the residue, reducing waste and increasing Glycerine recovery. Pharmaceutical-grade Glycerine, the main product, undergoes purification through adsorption on activated carbon beds to bleach it, diverting light impurities to technical-grade Glycerine. These methods not only ensure efficient Glycerine separation but also minimize waste and improve overall recovery of this valuable substance.
Major Applications of Glycerine
Cosmetics & Personal Care:
Glycerin offers significant benefits for skin health due to its properties as a humectant, solvent, and lubricant, making it a valuable ingredient in skincare formulations. Its capacity to reduce moisture loss helps in keeping the skin hydrated for extended periods compared to other moisturizers. Studies affirm Glycerin's potent moisturizing effects, establishing it as a robust and efficient humectant, particularly beneficial for dry skin. Glycerin holds significant utility in personal care products, serving as a versatile ingredient due to its properties as a humectant, solvent, lubricant, and alternative to sorbitol, another sugar alcohol used as a sweetener. It finds widespread application in various products including toothpaste, mouthwash, skin care formulations, deodorants, soaps, and baby care products. In these formulations, Glycerin contributes to moisturization, texture enhancement, and overall product efficacy, making it a staple in the personal care sector.
Food & Beverages:
Glycerin plays a multifaceted role in the food industry, commonly found in processed, packaged, and frozen foods where it serves various purposes. Acting as an emulsifier, it stabilizes ingredients and prevents separation, while also serving as a sugar substitute, adding sweetness to food products. Its humectant properties help preserve foods by preventing moisture loss, while also acting as a solvent for food coloring and flavors, aiding their dispersion. Additionally, Glycerin acts as a softening agent in candies, cakes, and meat/cheese casings, contributing to their texture and quality.
Pharmaceuticals:
Glycerin serves as a vital component in the production of several pharmaceutical items, including suppositories, where it forms the base for medications enclosed in solid Glycerin pieces. Additionally, Glycerin is utilized in the manufacturing processes of cough medicines, gel capsules, certain medications, and specific types of anesthetics, playing essential roles in formulation consistency, delivery methods, and therapeutic efficacy within the pharmaceutical industry.
Market Outlook
The primary driver of market growth is the widespread adoption of Glycerin in personal care and cosmetics. It is extensively utilized in various products like toothpaste, soaps, shaving creams, and skin and hair care items to enhance smoothness and lubrication. Glycerin's ability to prevent moisture loss from these products makes it a valuable component, employed as denaturants, fragrance ingredients, oral care agents, hair conditioning agents, and skin protectants.
Glycerine Main Players
Significant companies in the Global Glycerine market are The Procter & Gamble Company, Dow Chemical, Renova S.A., Emery Oleochemicals, Vantage Specialty Chemicals, Louis Dreyfus Company, General Lagos, BOJAGRO S.A., Vance Group Ltd., Owensboro Grain Company, The Vegetables Vitamins Foods Company Pvt. Ltd., PMC Biogenix, Inc., Thai Glycerine Co., Ltd., and Others.
Conclusion:
Glycerine finds extensive utilization across diverse industries, notably in food and beverages as a sweetener, and in medicinal and cosmetic formulations as an emollient. Its water-absorbing properties, attributed to hydroxyl groups, make it invaluable in these applications. With recognized antibacterial and antiviral qualities, Glycerine is a common ingredient in skincare products, aiding in skin healing and smoothness. In the pharmaceutical industry, it features prominently in various medications, including cough syrups, expectorants, and allergen immunotherapies. Additionally, nitroGlycerin stands out as a prevalent treatment for chronic angina. Glycerin’s versatility extends to topical treatments for conditions like psoriasis and wounds. Market drivers for Glycerine include the healthcare, cosmetic, and medical industries, where its moisturizing properties are leveraged to treat skin ailments and promote hygiene awareness. This increasing demand is expected to fuel the global Glycerine market in the coming years, driven by its antimicrobial properties and diverse applications across multiple sectors.
#glycerine#glycerineprices#glycerinemarket#glycerinenews#glycerinedemand#glycerinesupply#glycerinepricetrend#glycerinepriceforecast#glycerinemarketprice#priceofglycerine
1 note
·
View note
Text
Unveiling the Potential of BMK Ethyl Glycidate: Understanding its Chemical Structure and Versatile Applications
BMK Ethyl Glycidate, a compound known for its versatility, has attracted significant attention across various industries due to its distinct properties and wide-ranging applications. This article provides a comprehensive exploration of BMK Ethyl Glycidate's chemical composition, synthesis methods, and its extensive uses in pharmaceuticals, fragrances, and beyond. Additionally, it delves into regulatory considerations surrounding its usage and discusses potential future prospects.
Introduction: BMK Ethyl Glycidate, also referred to as ethyl 3-oxo-4-phenylbutanoate, is a notable chemical compound derived from glycidic acid and ethyl alcohol. With its characteristic fruity scent and molecular formula C12H14O5, BMK Ethyl Glycidate has emerged as a prominent player in various industries due to its diverse applications and favorable chemical properties.
Chemical Composition: The synthesis of BMK Ethyl Glycidate involves esterification, where glycidic acid reacts with ethanol under acidic conditions, leading to the formation of an ester linkage. This results in the molecular structure of BMK Ethyl Glycidate, comprising a phenyl group attached to a butanoate backbone, with an oxo group positioned at the third carbon atom.
Synthesis Methods: Numerous methods exist for synthesizing BMK Ethyl Glycidate, each employing different reaction conditions and catalysts. Common methods include refluxing glycidic acid and ethanol with a strong acid catalyst like sulfuric acid or hydrochloric acid. Alternatively, transesterification reactions using ethyl acetate as a starting material have also been employed for BMK Ethyl Glycidate synthesis.
Applications: In the pharmaceutical sector, BMK Ethyl Glycidate serves as a crucial precursor for synthesizing various pharmaceutical compounds, including benzyl methyl ketone (BMK), which is an important intermediate in the production of pharmaceuticals such as amphetamines and other psychoactive substances. Additionally, its fruity aroma makes it a desirable ingredient in perfumes and flavoring agents within the fragrance industry.
Regulatory Considerations: Due to its association with the synthesis of controlled substances, particularly amphetamines, BMK Ethyl Glycidate is subject to stringent regulatory controls in many jurisdictions. Authorities closely monitor its production, distribution, and sale to prevent illicit use in drug manufacturing. Compliance with regulatory requirements is essential for companies involved in the production and distribution of BMK Ethyl Glycidate to ensure legal and ethical practices.
Future Perspectives: Despite regulatory challenges, the demand for BMK Ethyl Glycidate is expected to remain robust due to its indispensable role in pharmaceutical and fragrance industries. Ongoing research efforts aim to develop more efficient synthesis methods, explore novel applications, and address regulatory concerns to ensure responsible use and foster continued innovation.
Conclusion: In conclusion, BMK Ethyl Glycidate emerges as a versatile compound with diverse applications spanning pharmaceuticals, fragrances, and various other sectors. A thorough understanding of its chemical structure, synthesis methods, applications, and regulatory considerations is essential for researchers, industry professionals, and regulatory authorities alike. Continued exploration of BMK Ethyl Glycidate's properties and applications holds promise for further advancements while upholding ethical and regulatory standards.
0 notes