#baculovirus
Photo
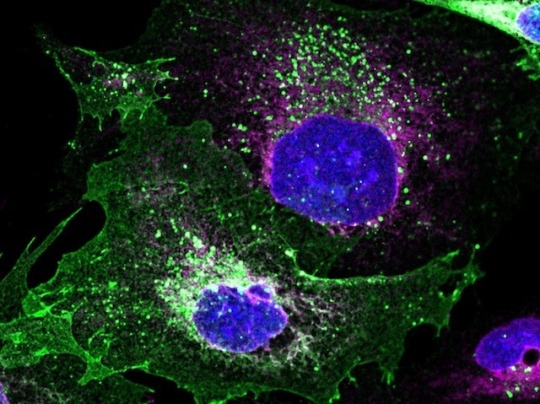
Repair Kit Delivery
If you have problems at a factory, it’s better to fix the machinery than repair each item produced. This is the philosophy of gene therapy, which aims to solve errors in DNA and subsequent protein production rather than manage the eventual symptoms. Steroid-resistant nephrotic syndrome is a debilitating genetic condition in which the protein podocin, which usually sits on the surface of kidney cells, becomes stuck inside. Many gene therapies use viruses to transport material into cells, but many of these are of limited size, so are like trying to manoeuvre a crane in place with a bicycle. A new study modified the harmless baculovirus to have large capacity, and used it to smuggle replacement DNA sequences, molecular scissors, and guiding nucleic acids into diseased cells (pictured). Podocin (green) was restored to the cell surface, addressing the problem at its source and proving the technique’s potential for many applications.
Written by Anthony Lewis
Image from work by Francesco Aulicino and colleagues
BrisSynBio Bristol Synthetic Biology Centre, Biomedical Sciences, School of Biochemistry, University of Bristol, Bristol, UK
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Nucleic Acids Research, July 2022
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#podocin#nephrotic syndrome#kidneys#crispr#gene editing#molecular scissors#baculovirus#nucleic acids#gene therapy#immunofluorescence
9 notes
·
View notes
Text
@ghoulhugs submitted: CW for dead bug and really gross details.

I had a question about what's happening to the caterpillars in my backyard. The other day, I accidentally left an empty cup outside. When I went to get it, there was a caterpillar around the rim. I poked at it to see about relocating the caterpillar, but it... melted against my finger. It turned from a caterpillar to goo with hardly any pressure being applied. I was disturbed, but didn't think too much about it.
Today, I was outside and saw this little caterpillar on the side of a panel we have outside. This time, I used a flimsy blade of grass to very gently prod it when I didn't see it moving. This was the result. It just completely melted.
I would think it was a molt, but it had actual guts. A web search isn't helping because the results just want to talk about how they liquify in the cocoon, which I don't think is related to this? Is it possible some disease or chemical is doing this to them? 😭
I would say most likely a virus or bacteria. There are a few of them that will make caterpillars turn into goo, but this one looks a lot like baculovirus to me. It makes the caterpillar climb high up and then die and liquefy, dripping virus down onto whatever's below it to keep spreading it to other bugs.
151 notes
·
View notes
Text
Viral Vector Gene Delivery Market: Advancements, Opportunities, and Future Trends
Market Overview
Viral vector is the most sought-after way to transfer genes to specific cell types or tissues and manipulate them to express therapeutic genes. Currently, there are several types of viruses, such as adenovirus, adeno associated virus, baculovirus and retrovirus, which are being investigated in order to deliver the genes to required cells. This process can provide permanent as well as transient transgene expression.
Market Dynamics
Viral vectors are primarily used in the development of various gene therapies and are showing great promise in disease landscape. Moreover, during the unprecedented times of COVID-19, the development of adenovirus-vector vaccine has helped to mitigate the dangerous risks associated with the pandemic.?
Since the viral vector-based gene therapies have shown promising results in the past, the developers are exploring its full potential to reach to its long-term success. With the ongoing rise in demand, the service providers are continuously expanding their facilities to meet the numbers. In August 2022, Thermofischer has expanded its cell and gene therapy capability by opening the new viral vector manufacturing facility in Massachusetts, US.
In June 2022, VIVEbiotech has completed the acquisition of its lentiviral vector manufacturing capabilities to support the customers throughout the development cycle.
In June 2022, Avid Bioservices expanded its viral vector focused facility with 53,000 sq. ft. area to address the demand of viral vector production at commercial scale.
The increasing prevalence of various genetic disorders and target diseases increased funding availability for development of gene therapy, research efforts in the field of viral vector-based cell and gene therapies, and increasing efficacy of viral vectors in delivering gene therapy are fueling growth in the global viral vectors manufacturing market.
Click here for full report:
Challenges
The ongoing challenge, which is linked with its ability to pass the immune system and high number of doses are some of the limiting factors delaying the success of such therapies. Further, the quality of transgene expression plays another crucial role for the desired clinical effect. The combinatorial factors demand innovations at the R&D scale, which will gain traction in investment from various organizations.
In June 2022, NSW Government of Australia allocated USD 101.4 million to the commercial facility of viral vector manufacturing. This clearly indicates the need to improve the viral vector-based therapy due to its significant potential to cure multiple indications.??
Development Landscape:
Biotech and pharmaceutical businesses are adopting both organic and inorganic growth strategies to advance their pipeline and gain competitive edge in the market over the peers.
In April 2022, ProteoNic and NecstGen announced a collaboration, wherein the respective players will utilize their proprietary platforms to advance the AAV and LV viral vector manufacturing technology.
Till now, only eight viral vector-based gene therapies have been approved by the US FDA, which were developed using three viral vectors- adeno associated virus, lentivirus and herpes simplex virus.
As of September 2022, over 145 clinical trial studies are going on with over 10,000 patients worldwide. The exploratory pipeline of viral vector-based therapy continues to grow across the indications and targets with promising results.
Click here for free sample report:
Key players:
Lonza (Switzerland), Merck (Germany), Oxford BioMedica (UK), CGT Catapult (UK), Cobra Biologics (UK), uniQure (Netherlands), FUJIFILM Diosynth Biotechnologies (US), and Spark Therapeutics (US)
Click here for full report:
https://www.pharmanucleus.com/reports/viral-vector-gene-delivery
Upcoming players:
TBD
Regional Analysis:
North America?is anticipated to capture the highest share of this market over the forecast period (2022-2027), with U.S. accounting for the maximum contribution. Presence of most number of manufacturing facilities and key players, some of the best research universities and an encouraging start-up eco system provides an ideal environment for research leading to more successful assets in pipeline. Macro-economic factors such as large population and growing economy, high prevalence of cancer and infectious diseases, better access to insurance, availability of crowd funding and support from various stakeholders’ fuels US as a commercially attractible market.
In 2019, the New Jersey Innovation Institute signed an agreement with Pall Corporation to develop a BioPharmaceutical Innovation iLab to help fix the viral vector shortage using continuous manufacturing.
Thermo Fisher Scientific opened a cGMP manufacturing facility in Plainville, US which helped double the company’s commercial viral vector capacity?and?meet rising demand for the development and manufacturing of gene therapies and vaccines.
Europe?also remains a favourable region on par with the North American region. Presence of highly skilled researchers, well-developed R&D ecosystem, support from government agencies and regulatory bodies are the major driving factors in Europe.
In 2021, Yposkesi announced that its bioproduction project has been selected for the French government’s “Plan de Relance” initiative, which is an economic stimulus package aimed at making the nation’s bioproduction industry strategically more robust and economically resilient is investing USD6 million in its “Boost” project to develop a new generation 1000-L, GMP platform with optimized manufacturing and control processes for efficient viral vector production from the thawing of cells up to the aseptic filling of the end product.
Asia-Pacific?region is expected to pick up pace over the next few years due to favourable regulatory climate, increasing investments to drive market access in key markets such as Japan, China, Australia, Singapore and India. The market is focusing on Asia-Pacific as a destination for outsourcing and trying to gain foothold through mergers, collaborations, and strategic acquisitions.
In 2022, National Cancer Center Japan and GenScript ProBio entered into research collaboration agreement for a plasmid and lentiviral vector CMC development to develop plasmid and lentiviral vector for cell therapy to aim on FDA acceptance of IND applications.
In 2022, VectorBuilder, a CDMO announced an investment of USD 500m for China viral vector plant. The campus will include 30 production suites designed to manufacture lentivirus, plasmids, messenger RNA (mRNA), adeno-associated virus (AAV), cell lines and other types of viral and non-viral vectors.
#viral vector gene delivery#gene therapy#biotechnology#vector systems#viral vectors#genetic medicine#viral vector market#gene delivery trends
0 notes
Text
Transcriptomics and interactomics during the primary infection of an SfNPV baculovirus on Spodoptera frugiperda larvae
Pubmed: http://dlvr.it/T05K1s
0 notes
Text
Research Technician - Viral Tools
HHMI Janelia Research Campus
See the full job description on jobRxiv: https://jobrxiv.org/job/research-technician-viral-tools/?feed_id=47071
#ScienceJobs #hiring #research
Ashburn #UnitedStatesUS #ResearchTechnician
0 notes
Text
ANGUS ACQUIRES EXPRESSION SYSTEMS, LLC
https://questlation.com/prnewswire/7d81864e502a289b2ae115fc0dbc11ab/?feed_id=18584&_unique_id=64146c6c62d65
0 notes
Text
Fwd: Postdoc: WageningenU.BehaviouralManipulation
Begin forwarded message:
> From: [email protected]
> Subject: Postdoc: WageningenU.BehaviouralManipulation
> Date: 22 February 2023 at 05:42:41 GMT
> To: [email protected]
>
>
> We are recruiting a postdoc (2 years) to study viral manipulation
> of caterpillar behaviour. Parasites, including viruses, are able to
> manipulate the behaviour of their hosts to increase their transmission
> probability. The goal of this study is to unravel the molecular
> mechanisms behind behavioural manipulation by viruses in insects,
> for which caterpillars infected with baculoviruses will be used as a
> model. Infected caterpillars climb to elevated positions prior to death,
> a phenomenon known as tree-top disease. In this project you will apply a
> transcriptomic approach to reveal differences in gene expression patterns
> between virus-infected caterpillars under conditions triggering (light)
> and non-triggering (dark) tree-top disease. These data will provide
> information about virus and insect signal transduction pathways and
> effector genes that lead to altered host behaviour. In addition, small
> RNAs will be sequenced to look into the role of micro RNAs (miRNAs). A
> number of differentially regulated genes or miRNAs will be selected for
> follow-up studies (including both molecular and behavioural analyses)
> to confirm and specify their role in parasitic manipulation. The
> project will also aim to investigate tree-top disease in multiple
> caterpillar-baculovirus systems, to identify possible homologies and
> infer the evolutionary history of viral manipulation.
>
> This postdoc position is embedded in the group of Dr. Vera
> Ros within the Laboratory of Virology, Wageningen
> University & Research (The Netherlands). Our research
>
0 notes
Text
Hsp70 Human Protein (Baculovirus expressed)
Hsp70 Human Protein (Baculovirus expressed)
Catalog number: B2012571
Lot number: Batch Dependent
Expiration Date: Batch dependent
Amount: 50 ug
Molecular Weight or Concentration: 71.7 kDa
Supplied as: Solution
Applications: molecular tool for various biochemical applications
Storage: -20°C
Keywords: Hsp70 (human recombinant, baculovirus expressed)
Grade: Biotechnology grade. All products are…

View On WordPress
0 notes
Text
Novavax vaccine contains 1 mcg of insect (the fall armyworm) and baculovirus proteins and a bit of their DNA too, which is injected into you with each dose
0 notes
Text
Definitive host of taenia solium pdf
DEFINITIVE HOST OF TAENIA SOLIUM PDF >>Download
vk.cc/c7jKeU
DEFINITIVE HOST OF TAENIA SOLIUM PDF >> Read Online
bit.do/fSmfG
T. saginata. Ingested by specific intermediate host. Scolex released. Dr. Eva Askar. Nonnoron. Attaches lo intestine ܀ Develops into adult ! Development of an Enzyme-Linked Immunoelectrotransfer Blot (EITB) Assay Using Two Baculovirus Expressed Recombinant Antigens for Diagnosis of Taenia solium cysticercus ﻤﺎ Taenia solium Taenia saginata اﻟﻨﻬﺎ اﻟﻤﻀ ﻒ :Definitive host. Human Intermediate Pigs Cattle, sheep :host & various herbivores 2- Taenia Solium Host: Man (definitive host) and Cattle (intermediate host) Life cycle it is similar to that of except human becomes infected by3- The intermediate host of Schistosoma haematobium is that of Schistosoma mansoni is 2- Pigs acts as intermediate host of Taenia solium. Taenia saginata excretory canal Tapeworm oy and tapeworm segment. Dr. Eva Askar. Cysticercus intermediate host. Cow. T. saginata. whose man is the only definitive host. Cysticercosis, an infection caused by the larval form of Taenia solium is a parasitic disease that often affects Taenia Saginata اتانيجاسانيت ةدود. قيرط نع ةباصإلا متتو . Life cycle ةايحلا ةدود Taenia Solium ميلوس اينيت ةدود Taenia Saginata اتانيجاس اينيت ةدود.
https://www.tumblr.com/pocihexitaq/698174468203429888/al-design-workshop-%D9%83%D8%AA%D9%8A%D8%A8, https://www.tumblr.com/pocihexitaq/698174754181496832/patient-safety-and-quality-an-evidence-based-for, https://www.tumblr.com/pocihexitaq/698174179514712064/starbucks-coffee-and-tea-resource-pdf-%D9%83%D8%AA%D9%8A%D8%A8, https://www.tumblr.com/pocihexitaq/698174593146519552/como-sutura-una-herida-pdf-writer, https://www.tumblr.com/pocihexitaq/698174179514712064/starbucks-coffee-and-tea-resource-pdf-%D9%83%D8%AA%D9%8A%D8%A8.
0 notes
Text
U.S. Protein Expression Market Size, Share and Demand Forecast to 2027
The U.S. Protein Expression Market size will reach USD 3.2 billion by 2027 and is expected to develop at a CAGR of 11.8% during the forecast period (2021-2027), according to VynZ Research. For the forecast year 2021-2027, as well as the historical period 2015-2020, the U.S. Protein Expression Market has been studied.
The research report provides a comprehensive and insightful examination of the U.S. Protein Expression Market and includes market analysis on segmentation, dynamics, competition, and regional development. It considers the U.S. Protein Expression Market's CAGR, value, volume, revenue, production, consumption, sales, manufacturing cost, pricing, and other significant parameters. The forecasts in the report are based on well-established research methodology and assumptions.
Get the sample copy of the Market Research @ https://www.vynzresearch.com/healthcare/us-protein-expression-market/request-sample

Individual strategies were examined in the U.S. Protein Expression Market study, followed by business profiles of U.S. Protein Expression Market providers. The study includes an 'Industry Landscape' section that provides readers with a comprehensive view and firms’ market share analysis of major industry players in the Market.
Most of the major players in the U.S. Protein Expression Market are profiled in the report. The strengths and weaknesses, business developments, recent innovations, mergers and acquisitions, expansion plans, U.S. footprint, market presence, and product portfolios of key market competitors are all covered in the company profiling section.
The following are some of the major and developing players in the Market:
New England Biolabs, Inc.
Thermo Fisher Scientific Inc.
QIAGEN N.V.
Takara Bio Inc.
Lonza Group Ltd.
Merck KGaA
Promega Corporation
Genscript Biotech Corporation
Agilent Technologies Inc.
Bio-Rad Laboratories, Inc.
Breakdown of The Segments:
The U.S. Protein Expression Market is segmented by Product and Services, System channel, Application, and End-user in this study. This segmentation aids executives in planning their products and budgets depending on each segment's expected growth rates.
By Product & Services
Expression vectors
Reagents
Competent cells
Instruments
Services
By Systems Channel
Prokaryotic expression systems
Yeast expression systems
Mammalian cell expression systems
Cell-free expression systems
Algal-based expression systems
Insect cell expression systems
coli systems
Others
Saccharomyces systems
Lactis systems
Pichia systems
Others
Chinese hamster ovary system
Others
Baculovirus system
Others
By Application
Therapeutics
Research
Industrial
By End-User
Academic research institutes
Contract research organizations
Pharmaceutical and biotechnology companies
Others
Geographical Viewpoint:
The research overview provides the major industry trend and the US market's predicted volume based on the regional analysis. The elements that have driven and hampered the market's expansion are also mentioned in this market research analysis. The study is also equipped with the most advanced and effective techniques for gathering, recording, estimating, and evaluating market data.
FAQ
Who are the most dominant players in the US market, and what elements are assisting them in gaining a competitive advantage?
What are the strategies adopted by the key industry players to gain traction in the industry?
By the end of the forecast period, what will the market size and growth rate?
What are the biggest U.S. Market trends that are influencing market growth?
In the US market, which segment had the biggest revenue share?
Explore More Reports by VynZ Research:
Global Solar Energy Market – Analysis and Forecast (2022-2030)
Global Power Rental Market - Analysis and Forecast (2022-2030)
Global Energy Storage System Market – Analysis and Forecast (2022-2030)
Global Variable Frequency Drive Market – Analysis and Forecast (2022-2030)
About VynZ Research
VynZ Research is a global market research firm offering research, analytics, and consulting services on business strategies. VynZ have a recognized trajectory record and our research database is used by many renowned companies and institutions in the world to strategize and revolutionize business opportunities. The company focuses on providing valuable insights on various technology verticals such as Chemicals, Automotive, Transportation, Energy, Consumer Durables, Healthcare, ICT and other emerging technologies.
#U.S. Protein Expression Market#U.S. Protein Expression Market share#U.S. Protein Expression Market scope#U.S. Protein Expression Market value
0 notes
Text
A fast and powerful method for the production of SARS-CoV-2 virus-like particles in insect cells
A fast and powerful method for the production of SARS-CoV-2 virus-like particles in insect cells
In a recent study published in Viruses, researchers presented the direct plasmid transfection method for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus-like particle (VLP) production in insect cells and applied the VLPs to evaluate the efficacy of monoclonal antibodies (mAbs) and vaccinated donor sera.
Study: Baculovirus-Free SARS-CoV-2 Virus-like Particle Production in Insect…

View On WordPress
0 notes
Text
Swine Vaccines Market Size Worth $2.09 Billion By 2028

The global swine vaccines market size is expected to reach USD 2.09 billion by 2028, registering a CAGR of 4.9% over the forecast period, according to a new report by Grand View Research, Inc. Increasing prevalence of diseases, a surge in the demand for animal protein, and growth in R&D expenditure for vaccines innovation are driving the market growth. Porcine Parvovirus (PPV), Porcine Reproductive and Respiratory Syndrome (PRRS), Porcine circovirus type 2 (PCV2), Foot & Mouth Disease, Classical Swine Fever, and others remain to be extremely prevalent diseases in pig farms affecting economic losses.
The COVID-19 pandemic directly or indirectly impacted the business, results of operations, and financial conditions of market players. The Animal Health business was confronted with various production and supply bottlenecks because of the COVID-19 pandemic; this consists of delays in the delivery of raw materials and active ingredients and capacity constraints in freight transport (especially air and sea freight). While on the other side, the industry has expanded its research at an unprecedented pace in animal health. Thereby, the COVID-19 pandemic significantly impacted the market growth.
Free Report Sample: https://www.grandviewresearch.com/industry-analysis/swine-vaccines-market-report/request
In addition, the growing investments by key players to develop advanced products for better diagnosis are likely to propel market growth. For instance, in May 2020, Indian Immunologicals Ltd. introduced the Classical Swine Fever vaccine. Stating that it has introduced the product under the brand name ‘Raksha Class’. This is a unique cell culture technology-based vaccine developed in cooperation with the Indian Veterinary Research Institute, Bareilly.
Key players are involved in strategic initiatives, such as joint ventures, mergers, acquisitions, and new product launches to gain a higher market share. For instance, in February 2020, the new vaccine created by ICAR, Indian Veterinary Research Institute (IVRI), based in Uttar Pradesh, was much cheaper than an already existing one. It costs only Rs 2 for each dose over the present vaccine’s rate of Rs 15-20 for each dose and an imported Korean vaccine rate of Rs 30 for each dose.
Swine Vaccines Market Report Highlights
The inactivated vaccines segment dominated the market in 2020 owing to its easy availability and low cost over others
A recombinant vaccine is expected to register the fastest CAGR over the forecast period owing to the benefits, such as enhanced effectiveness, technology advancement, and increased long-standing prevention
The Porcine circovirus type 2 segment dominated the market in 2020 due to the increased prevalence of the disease. Moreover, this is the quickly mutating of all single-stranded DNA (ssDNA) viruses, therefore it has a great mutation and recombination rate
Asia Pacific is expected to register the fastest CAGR over the forecast period due to the growing cases of infectious diseases in swine, increasing awareness about animal health, rising demand for animal protein, and rapid urbanization
Key Companies & Market Share Insights
The market is highly competitive and marked by the existence of several small- and large-scale manufacturers. These players are constantly involved in strategic initiatives, such as new product launches, regional expansions, joint ventures, mergers, and acquisitions, to gain deeper market penetration. For instance, in February 2021, Algenex SL and Virbac proclaimed that they have entered an international licensing agreement for the development and commercialization of a CrisBio-based vaccine in a key swine indication.
The vaccine will be created together and manufactured using CrisBio, Algenex’s patented protected Baculovirus vector-mediated expression platform, which leverages the power of insects to function as natural single-use bioreactors. In June 2020, Boehringer Ingelheim received a New Veterinary Drug Registration Certificate from the Ministry of Agriculture and Rural Affairs of China for Ingelvac CSF MLV. This is the first CSF live vaccine developed jointly by Chinese research institutes and multinational companies. Some of the prominent players in the global swine vaccines market include: Merck Animal Health, Ceva, Zoetis, Boehringer Ingelheim GmbH, Elanco, Indian Immunologicals Ltd., BiogénesisBagó, Phibro Animal Health, KM Biologics, HIPRA
Buy Full Report: https://www.grandviewresearch.com/industry-analysis/swine-vaccines-market-report
0 notes
Text
U.S. Protein Expression Market to Eyewitness Robust Expansion by 2027
VynZ Research provides market overview of the current environment as well as the industry's future growth in the U.S. Protein Expression market. It uses data from annual reports, product literature, industry developments, and other sources to conduct a quantitative market study. The future growth prospects of the industry are based on a rapid quantitative and qualitative assessment of data from various sources.
The U.S. Protein Expression market is projected to reach USD 3.2 billion by 2027, witnessing a CAGR of 11.8% during the forecast period 2021-2027.
To assist companies in developing their business plans, the research examines the major factors influencing the growth of the U.S. Protein Expression market, also including Drivers, Constraints, Governmental Policies, Opportunities, Challenges, a Constructive Approach, and Market Economic Expansion Strategies.
Get a free sample copy of this market research @ https://www.vynzresearch.com/healthcare/us-protein-expression-market/request-sample
The influence of recent market disruptions such as the Russia-Ukraine war and Covid-19 on the market will be examined in this research report.
Segments Defined:
The research report divides the market into segments based on geography, by Product & Services, Systems Channel, Application and End-User During the forecast period of 2021 to 2027, each category gives data on production and consumption. Understanding the segments aids in determining the importance of various market growth variables.
Market segmentation:
Product & Services Insight
Expression vectors
Reagents
Competent cells
Instruments
Services
Systems Channel Insight
Prokaryotic expression systems
E. coli systems
Others
Yeast expression systems
Saccharomyces systems
K. Lactis systems
Pichia systems
Others
Mammalian cell expression systems
Chinese hamster ovary system
Others
Cell-free expression systems
Algal-based expression systems
Insect cell expression systems
Baculovirus system
Others
Application Insight
Therapeutics
Research
Industrial
End-User Insight
Academic research institutes
Contract research organizations
Pharmaceutical and biotechnology companies
Others
Region and Country Specific Defined:
Thorough research of certain regions and their related countries is conducted to guarantee that the comprehensive detailing of the U.S. Protein Expression market's footprint and sales demographics are captured with accuracy, allowing users to make the most of this data.
Competitive Scenario:
In order to obtain essential and critical industry data, the records of major market participants are analyzed. It provides vital information and status on industry players, as well as serves a useful source of advice for businesses and organizations. Porter's Five Forces analysis, SWOT analysis, PEST analysis, competitor landscape, development trends, and strategy analysis, are all covered in depth in the “U.S. Protein Expression market" research. Major breakthroughs, developments, mergers and acquisitions, and agreements with other important organizations have all been investigated.
Top players are:
New England Biolabs, Inc.
Thermo Fisher Scientific Inc.
QIAGEN N.V.
Takara Bio Inc.
Lonza Group Ltd.
Merck KGaA
Promega Corporation
Genscript Biotech Corporation
Agilent Technologies Inc.
Bio-Rad Laboratories, Inc.
FAQ
What are the trends and market dynamics along with market size and growth rate of the U.S. Protein Expression marketduring the forecast period?
What is the future impact of the U.S. Protein Expression marketand limits on the market?
What regions currently contribute the maximum share to the global market?
How the changed competitive dynamics can influence the U.S. Protein Expression market?
Who are the industry players in the U.S. Protein Expression market and what strategies are adopted by them to sustain in the market?
About Us: VynZ Research is a global market research firm offering research, analytics, and consulting services on business strategies. VynZ Research has a recognized trajectory record and our research database is used by many renowned companies and institutions in the world to strategize and revolutionize business opportunities. The company focuses on providing valuable insights on various technology verticals such as Chemicals, Automotive, Transportation, Energy, Consumer Durables, Healthcare, ICT and other emerging technologies.
0 notes
Text
Novavax autorizada por "emergencia"

Novavax autorizada por emergencia 18/07/22
1/2
Estimados miembros de Akasha Comunidad:
Sé que algunos miembros de la comunidad han pedido que hable acerca de la vacuna de la empresa Novavax (de nombre Nuvaxovid, Covovax o NVXCov2373). Ya lo he abordado en varios mensajes que ustedes pueden buscar, tanto en el índice semanal, como haciendo uso de la opción de búsqueda ('piquen' la lupa o los tres puntitos que salen arriba a la derecha, y escriban Novavax).
Me parece que no hay información ni conocimiento adicional desde que escribí esos mensajes que me lleven a tener otra postura al respecto de este producto.
A manera de resumen, los puntos principales que tomo en cuenta para considerar el posible beneficio de cualquier inoculación COVID-19 en este momento son:
1) La virulencia (daño ocasionado por el virus SARS-COV-2) es cada vez menor. Actualmente es de 0.2%, comparable con lo que ocasionan otros patogénos comunes, y - además - la media de edad de los que llegan a morir es de 82.5 años, mientras que entre más joven, menor es el riesgo hasta volverse prácticamente de cero.
2) Se sabe mucho más ahora que al inicio de este quilombo sanitario sobre cómo tratar a los pacientes afectados (y sobre cómo no tratarlos), lo que reduce aún más la tasa de letalidad de este virus.
3) Los análisis serológicos (aquí compartidos) han mostrado que más del 75% de la población en varios países del mundo ya contaba con anticuerpos generados por la infección. Estos, a diferencia de los que se generan en respuesta a las inoculaciones, sí son efectivos en la mayor parte de la gente que sea sana, para proteger de infectarse, transmitir al virus y enfermar.
4) Al igual que las inoculaciones de ARNm y las vectorizadas, las vacunas de Novavax solamente inducen una respuesta inmune adaptativa (medida en los estudios como la generación de anticuerpos y, menos frecuentemente estudiada, una generación de linfocitos T citotóxicos específicos) contra Spike... Y eso es contra el Spike de la primera variante que circulaba al inicio de la pandemia. Ya ni siquiera está siendo el 'ganón' la variante omicron, así que ya se imaginarán si es sensato inducir respuestas inmunes contra algo tan obsoleto.
5) la proteína Spike es uno de los factores de virulencia de SARS-COV-2. Eso quiere decir que es uno de los responsables de los daños y eventos celulares que provocan la variedad de síntomas conocidos somos COVID-19. En otras palabras, estamos dando al cuerpo con este producto de Novavax, cierta cantidad de aquello que está asociado al daño. Aquí, a diferencia de Pfizer, Moderna, AstraZeneca, Johnson & Johnson, Sputnik y Cansino, al menos es solo esa cantidad la que tendremos en cada dosis (porque no contiene las instrucciones para que nuestras células sigan produciendo la proteína por semanas o meses). En ese sentido, a mi juicio, podría argumentarse que es menos amplio el abanico de riesgos, pero no es carente de ellos, como ningún producto farmacológico lo es. Y si no se trata de un patógeno de alta virulencia o de una enfermedad contra la que no hubiera tratamiento, entonces, ¿para qué exponernos a ese riesgo farmacológico?
6) Aunque se trata de una plataforma vacunal más conocida y usada por más tiempo que las de ARNm y las vectorizadas, el producto también utiliza nanopartículas lipídicas que rodean a las proteínas Spike. Estas proteínas Spike fueron 'cosechadas' en células Sf9 de insecto, que fueron antes manipuladas para que fabricaran muchas copias de la proteína luego de haber sido infectadas (se dice transfectadas en el argot científico) por un baculovirus (una familia de virus de insectos) que, a su vez, fue modificado genéticamente para contener la secuencia de la proteína Spike (en otras palabras, hacen un 'Frankenstein' de un virus que infecta insectos, para poder producir millones de copias de la proteína deseada en un bioreactor que contiene células de insecto).
(Continúa en 2/2)
0 notes
Text
Preclinical along with Clinical Proof of Restorative Real estate agents regarding Verteporfin-Induced Side-line Neuropathy
Methods: Anti-human ATX monoclonal antibodies had been manufactured by immunization regarding recombinant man ATX indicated inside a baculovirus technique. An inummoassay for your quantitative resolution of ATX was established, along with human being solution trials had been assayed. Results: The particular within-run as well as between-run detail, interference, recognition restrict, and linearity scientific studies have been adequate. The particular central 92 percentile research period of time to the serum ATX antigen awareness in healthful themes ended up being 0.468-1.134 mg/l (n = One-hundred-twenty) and was strongly corrleated with the solution lysoPLD activity., Your ATX concentration ended up being considerably (p<3.001) larger ladies (0.625-1.323 mg/l) compared to guys (Zero.438-0.914 mg/l). The particular solution ATX concentrations of mit were increased in patients along with long-term liver organ ailments and also diminished in postoperative prostate cancer patients nevertheless are not transformed inside nephrosis individuals #Link# . Thus, solution ATX antigen concentrations could be accustomed to discriminate these hypoalbuminemia conditions. Conclusions: The current ATX antigen assay might be helpful for scientific lab testing. (d) '07 Elsevier B.Versus. Almost all privileges set aside.Mammalian hibernation is associated with broad alternative throughout heartbeat, blood circulation, along with fresh air supply for you to flesh and it is utilized as one particular associated with natural ischemia/reperfusion. Inside non-hibernators, ischemia/reperfusion is typically associated with oxidative stress yet hibernators seem to take care of possible oxidative destruction by improving de-oxidizing safeguarding within an anticipatory method. The present study examines the role with the Nrf2 transcribing element in the particular regulating antioxidant defense in the course of hibernation. Nrf2 mRNA as well as health proteins term ended up improved in decided on organs involving 13-lined floor squirrels, Spermophilus tridecemlineatus in the course of hibernation. Moreover, Nrf2 proteins throughout heart ended up being raised through One particular.4-1.Your five crease at multiple stages over the torpor-arousal round including through admittance, long lasting torpor, and also early excitement. Levels came back in order to euthermic beliefs any time squirrels were entirely turned on within interbout. Health proteins #Link# levels of decided on downstream focus on family genes below Nrf2 management had been in addition assessed via immunoblotting in the torpor-arousal never-ending cycle within cardiovascular. Cu/Zn superoxide dismutase as well as aflatoxin aldehyde reductase quantities more than doubled in the course of accessibility directly into torpor and then progressively decreased slipping to manipulate amounts or under in totally excited pets. Heme oxygenase-1 also showed precisely the same development. This means that a role pertaining to Nrf2 within money antioxidising protection needed for hibernation accomplishment. Cardiovascular nrf2 had been zoomed by simply PCR as well as sequenced. The deduced amino collection demonstrated high identity using the sequence business mammals #Link# but with chosen special alternatives (e.g., proline elements in roles 111 and 230) that may be necessary for conformational stableness in the protein from close to 3 degrees D entire body temps inside the torpid express.TLS/FUS (TLS) is a dual purpose necessary protein suggested as a factor in a wide range regarding cell procedures, such as transcription and also mRNA control, as well as in both cancer malignancy along with nerve condition.
0 notes