#pronucleus
Text
Wicked cutie is brought in butthole assylum for awkward therapy
Bbw latina gets smashed by BBC stranger
Comendo A Namorada Do Praticante De Bullying
Amateur footjob #70 nylon knee sock footjob, cum in sock
Gozando gostoso e gemendo muito
Lesbians are suffering to fuck with hot yang boyz and suck their dick
Fishball Suicide in stockings smokes a cigarette and shows her feet
Camiel is drinking his own morning pee
Vagina de mi vecina
Brunette Mandy May makes a titsfuck with her juicy melons for sperm shot
#anamorphous#cread#entei#Estell#salesman#transferor#Vahe#phattass#pocket-money#deperm#Jutish#pronucleus#pseudoembryonic#roed#misinterment#lumpenproletariat#methodicalness#surfboatman#senhores#undeliberate
0 notes
Note
So terf that can’t read you gonna give actual scientific evidence or not. Let’s see if you answer this or do you like hiding in the notes.
lol ok. Here’s medical journals and textbook citations to keep you up at night
“Overall, 95% of all biologists affirmed the biological view that a human's life begins at fertilization (5212 out of 5502). “
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3211703
"The development of a human being begins with fertilization, a process by which two highly specialized cells, the spermatozoon from the male and the oocyte from the female, unite to give rise to a new organism, the zygote."
[Langman, Jan. Medical Embryology. 3rd edition. Baltimore: Williams and Wilkins, 1975, p. 3]
"Embryo: An organism in the earliest stage of development; in a man, from the time of conception to the end of the second month in the uterus."
[Dox, Ida G. et al. The Harper Collins Illustrated Medical Dictionary. New York: Harper Perennial, 1993, p. 146]
"Almost all higher animals start their lives from a single cell, the fertilized ovum (zygote)... The time of fertilization represents the starting point in the life history, or ontogeny, of the individual."
[Carlson, Bruce M. Patten's Foundations of Embryology. 6th edition. New York: McGraw-Hill, 1996, p. 3]
http://www.lifeissues.net/writers/imb/imb_01lifebegins.html
"Human development begins after the union of male and female gametes or germ cells during a process known as fertilization (conception).
"Fertilization is a sequence of events that begins with the contact of a sperm (spermatozoon) with a secondary oocyte (ovum) and ends with the fusion of their pronuclei (the haploid nuclei of the sperm and ovum) and the mingling of their chromosomes to form a new cell. This fertilized ovum, known as a zygote, is a large diploid cell that is the beginning, or primordium, of a human being."
[Moore, Keith L. Essentials of Human Embryology. Toronto: B.C. Decker Inc, 1988, p.2]
The chromosomes of the oocyte and sperm are...respectively enclosed within female and male pronuclei. These pronuclei fuse with each other to produce the single, diploid, 2N nucleus of the fertilized zygote. This moment of zygote formation may be taken as the beginning or zero time point of embryonic development."
[Larsen, William J. Human Embryology. 2nd edition. New York: Churchill Livingstone, 1997, p. 17]
"Although life is a continuous process, fertilization is a critical landmark because, under ordinary circumstances, a new, genetically distinct human organism is thereby formed.... The combination of 23 chromosomes present in each pronucleus results in 46 chromosomes in the zygote. Thus the diploid number is restored and the embryonic genome is formed. The embryo now exists as a genetic unity."
[O'Rahilly, Ronan and M�ller, Fabiola. Human Embryology & Teratology. 2nd edition. New York: Wiley-Liss, 1996, pp. 8, 29. This textbook lists "pre-embryo" among "discarded and replaced terms" in modern embryology, describing it as "ill-defined and inaccurate" (p. 12}]
#immensely satisfied to be arguing so well I’m living in your head rent free. transphobes mad#the funny thing is I used to be more pro choice#and then I listened to the pro choice people and they’re all eugenecists#politics#my post
54 notes
·
View notes
Photo

Early stages of development in C. subdepressus under light microscope at 100X magnification depicting A) Activation of cortical reaction forming calcium barrier around cell periphery. Male pronucleus migration path to female pronucleus evident in microtubule aster pattern. B) First cleavage plane resulting in two daughter cells. Meridinal cleavage from animal to vegetal pole. C) Second meridinal cleavage occurring perpendicular to first plane. D) Third cleavage, plane equilateral, beginning of 4 cell stage development. Scale bar = 30 μm.
19 notes
·
View notes
Text


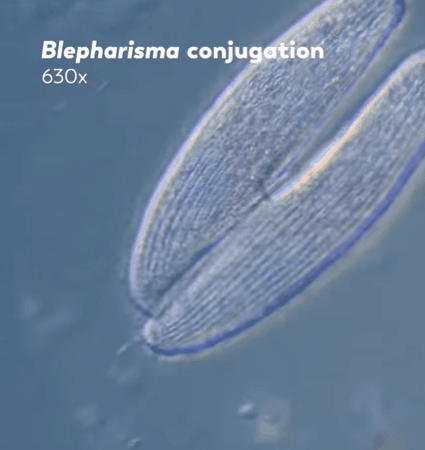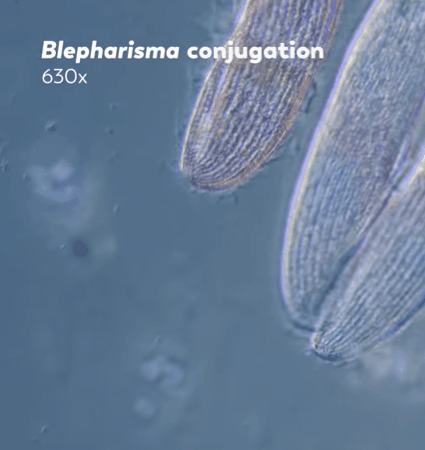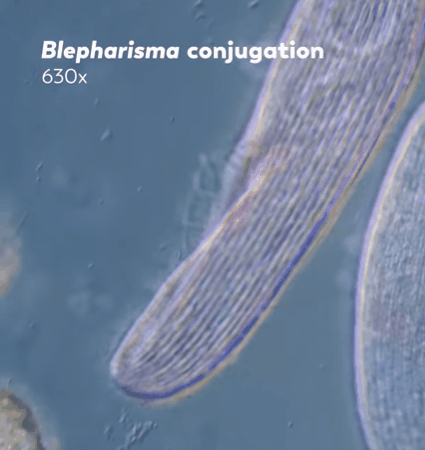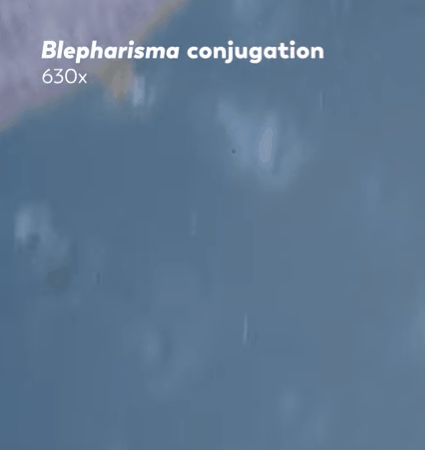



"The two ciliates will then exchange one of their pronuclei with the other through their connected mouths, and each will fuse their pronucleus with their partner's to create a zygotic nucleus. This new nucleus combines and rearranges the genetic material inherited from both parents, replacing the old macronucleus with this new remixed version."
Journey to the Microcosmos- Becoming Your Own Baby Through Conjugation
Images Originally Captured by Jam's Germs
#journey to the microcosmos#Becoming Your Own Baby Through Conjugation#scienceblr#science#biology#bioblr#microbiology#microorganisms#microbes#blepharisma#conjugation#hypotrich#ciliate#hypotrich ciliate#science aesthetic
60 notes
·
View notes
Text

Indeed, the thing inside you is well, ya know a human being. The definition of a human being is an:
“any individual of the genus Homo, especially a member of the species Homo sapiens.”
When you have an abortion, you lose an distinct member of the human species. You don’t just lose them, you kill them. You may argue that it’s not even alive, but even pro-choice biologists admit life begins at conception. After all, if it wasn’t would it be taking a toll on your body? Using your nutrients to survive? The definition of alive is...
”noun, plural: lives. (1) A distinctive characteristic of a living organism from dead organism or non-living thing, as specifically distinguished by the capacity to grow, metabolize, respond (to stimuli), adapt, and reproduce.”
If it wasn’t alive, would it really be able to develop into an fully developed newborn? No, clearly not. There’s also a reason why you often see the ”viability“ argument, arguing since it can’t survive outside the mother’s womb, she should be able to kill it. Which also obviously suggests it’s alive inside the womb. Also, see here:
”Life Begins at Fertilization
The following references illustrate the fact that a new human embryo, the starting point for a human life, comes into existence with the formation of the one-celled zygote:
"Development of the embryo begins at Stage 1 when a sperm fertilizes an oocyte and together they form a zygote."
[England, Marjorie A. Life Before Birth. 2nd ed. England: Mosby-Wolfe, 1996, p.31]
"Human development begins after the union of male and female gametes or germ cells during a process known as fertilization (conception).
"Fertilization is a sequence of events that begins with the contact of a sperm (spermatozoon) with a secondary oocyte (ovum) and ends with the fusion of their pronuclei (the haploid nuclei of the sperm and ovum) and the mingling of their chromosomes to form a new cell. This fertilized ovum, known as a zygote, is a large diploid cell that is the beginning, or primordium, of a human being."
[Moore, Keith L. Essentials of Human Embryology. Toronto: B.C. Decker Inc, 1988, p.2]
"Embryo: the developing organism from the time of fertilization until significant differentiation has occurred, when the organism becomes known as a fetus."
[Cloning Human Beings. Report and Recommendations of the National Bioethics Advisory Commission. Rockville, MD: GPO, 1997, Appendix-2.]
"Embryo: An organism in the earliest stage of development; in a man, from the time of conception to the end of the second month in the uterus."
[Dox, Ida G. et al. The Harper Collins Illustrated Medical Dictionary. New York: Harper Perennial, 1993, p. 146]
"Embryo: The early developing fertilized egg that is growing into another individual of the species. In man the term 'embryo' is usually restricted to the period of development from fertilization until the end of the eighth week of pregnancy."
[Walters, William and Singer, Peter (eds.). Test-Tube Babies. Melbourne: Oxford University Press, 1982, p. 160]
"The development of a human being begins with fertilization, a process by which two highly specialized cells, the spermatozoon from the male and the oocyte from the female, unite to give rise to a new organism, the zygote."
[Langman, Jan. Medical Embryology. 3rd edition. Baltimore: Williams and Wilkins, 1975, p. 3]
"Embryo: The developing individual between the union of the germ cells and the completion of the organs which characterize its body when it becomes a separate organism.... At the moment the sperm cell of the human male meets the ovum of the female and the union results in a fertilized ovum (zygote), a new life has begun.... The term embryo covers the several stages of early development from conception to the ninth or tenth week of life."
[Considine, Douglas (ed.). Van Nostrand's Scientific Encyclopedia. 5th edition. New York: Van Nostrand Reinhold Company, 1976, p. 943]
"I would say that among most scientists, the word 'embryo' includes the time from after fertilization..."
[Dr. John Eppig, Senior Staff Scientist, Jackson Laboratory (Bar Harbor, Maine) and Member of the NIH Human Embryo Research Panel -- Panel Transcript, February 2, 1994, p. 31]
"The development of a human begins with fertilization, a process by which the spermatozoon from the male and the oocyte from the female unite to give rise to a new organism, the zygote."
[Sadler, T.W. Langman's Medical Embryology. 7th edition. Baltimore: Williams & Wilkins 1995, p. 3]
"The question came up of what is an embryo, when does an embryo exist, when does it occur. I think, as you know, that in development, life is a continuum.... But I think one of the useful definitions that has come out, especially from Germany, has been the stage at which these two nuclei [from sperm and egg] come together and the membranes between the two break down."
[Jonathan Van Blerkom of University of Colorado, expert witness on human embryology before the NIH Human Embryo Research Panel -- Panel Transcript, February 2, 1994, p. 63]
"Zygote. This cell, formed by the union of an ovum and a sperm (Gr. zyg tos, yoked together), represents the beginning of a human being. The common expression 'fertilized ovum' refers to the zygote."
[Moore, Keith L. and Persaud, T.V.N. Before We Are Born: Essentials of Embryology and Birth Defects. 4th edition. Philadelphia: W.B. Saunders Company,
1993, p. 1]
"The chromosomes of the oocyte and sperm are...respectively enclosed within female and malepronuclei. These pronuclei fuse with each other to produce the single, diploid, 2N nucleus of the fertilized zygote. This moment of zygote formation may be taken as the beginning or zero time point of embryonic development."
[Larsen, William J. Human Embryology. 2nd edition. New York: Churchill Livingstone, 1997, p. 17]
"Although life is a continuous process, fertilization is a critical landmark because, under ordinary circumstances, a new, genetically distinct human organism is thereby formed.... The combination of 23 chromosomes present in each pronucleus results in 46 chromosomes in the zygote. Thus the diploid number is restored and the embryonic genome is formed. The embryo now exists as a genetic unity."
[O'Rahilly, Ronan and M�ller, Fabiola. Human Embryology & Teratology. 2nd edition. New York: Wiley-Liss, 1996, pp. 8, 29. This textbook lists "pre-embryo" among "discarded and replaced terms" in modern embryology, describing it as "ill-defined and inaccurate" (p. 12}]
"Almost all higher animals start their lives from a single cell, the fertilized ovum (zygote)... The time of fertilization represents the starting point in the life history, or ontogeny, of the individual."
[Carlson, Bruce M. Patten's Foundations of Embryology. 6th edition. New York: McGraw-Hill, 1996, p. 3]
"[A]nimal biologists use the term embryo to describe the single cell stage, the two-cell stage, and all subsequent stages up until a time when recognizable humanlike limbs and facial features begin to appear between six to eight weeks after fertilization....
"[A] number of specialists working in the field of human reproduction have suggested that we stop using the word embryo to describe the developing entity that exists for the first two weeks after fertilization. In its place, they proposed the term pre-embryo....
"I'll let you in on a secret. The term pre-embryo has been embraced wholeheartedly by IVF practitioners for reasons that are political, not scientific. The new term is used to provide the illusion that there is something profoundly different between what we nonmedical biologists still call a six-day-old embryo and what we and everyone else call a sixteen-day-old embryo.
"The term pre-embryo is useful in the political arena -- where decisions are made about whether to allow early embryo (now called pre-embryo) experimentation -- as well as in the confines of a doctor's office, where it can be used to allay moral concerns that might be expressed by IVF patients. 'Don't worry,' a doctor might say, 'it's only pre-embryos that we're manipulating or freezing. They won't turn into real human embryos until after we've put them back into your body.'"
[Silver, Lee M. Remaking Eden: Cloning and Beyond in a Brave New World. New York: Avon Books, 1997, p. 39]
0 notes
Text
Earlier this year a girl from my church asked me to help her prepare for an abortion debate her high school class was going to have the next day, so I quickly threw together some common pro-abortion points and the science and reasoning behind the pro-life position. Since then, a few other people have found it helpful, so I thought I would share it here as well.
_ _ _
1. Biologically, is a fetus a human?
Yes, it comes from two human parents and has human DNA. It is not a parasite because parasites are a different species than the host. It is not just a clump of cells or just a part of the parents like the mother's organs, the egg cell, or the sperm cell - because a fetus has unique DNA different from the mother's and father's DNA, making it an individual human organism.
"Although life is a continuous process, fertilization is a critical landmark because, under ordinary circumstances, a new, genetically distinct human organism is thereby formed.... The combination of 23 chromosomes present in each pronucleus results in 46 chromosomes in the zygote. Thus the diploid number is restored and the embryonic genome is formed. The embryo now exists as a genetic unity."
[O'Rahilly, Ronan and Muller, Fabiola. Human Embryology & Teratology. 2nd edition. New York: Wiley-Liss, 1996, pp. 8, 29.
(More quotes from scientists on the human life cycle and it's beginning at fertilization: https://www.princeton.edu/~prolife/articles/embryoquotes2.html )
2. Does it really matter if it is human or not? "It is so small / it doesn't look human yet / it won't feel pain / it won't know it is alive / etc."
Yes. Again, from a biological standpoint, a fetus is just one of the many stages of the human life. We all begin as zygotes (fertilized eggs), and rapidly begin growing and developing into embryos, then fetuses, then at 9 months we reach birth and begin the infant stage, continuing to become toddlers, adolescents, and finally adults. Just like it is wrong to say "a toddler is less of a human than an adult," it is also incorrect to say "an embryo is less of a human than a newborn infant." Just because someone is less developed than someone else does not make them less of a person.
By 6 weeks (1.5 months/ 42 days since fertilization) the human nervous system is established, and by 8 weeks (2 months) we have observed fetuses to physically react to and recoil from pain. https://lozierinstitute.org/fact-sheet-science-of-fetal-pain/
Sentience (or self-awareness), the most basic ability of something alive to understand its own existence - often first measured in animals by scientific researchers as the ability to recognize themselves in front of a mirror - doesn't develop in humans until months after birth. Everyone knows it would be silly to argue that it's okay to terminate the life of a newborn because it isn't self-aware yet, so logically it shouldn't be acceptable to argue that in the case of abortion either.
3. But abortion is legal / it's none of your business what other people choose!
We can see that many atrocities were legal at one point or another in history. Slavery used to be legal, child marriages used to be legal, many genocides (including the Holocaust) were legal in their countries. Just because something is legal does not make it morally justifiable.
It is always other people's business when injustice is happening. Is it wrong for a group of humans who have done nothing wrong to be intentionally killed, just because they are unwanted? Does that describe abortion? Then abortion is our business.
Bonus: A very technical scientific timeline of human development before birth: https://embryology.med.unsw.edu.au/embryology/index.php/Timeline_human_development . Pro-life is pro-science.
_ _ _
Because of the time restriction, as you can see I did not address the other common pro-abortion arguments centered on bodily autonomy/rape/health of the mother. Those can each be meticulously analyzed and flushed out, and have been many times, but for today I would just like my pro-choice friends to sincerely consider the question of "does your scenario justify ending an innocent person's life?" If you find yourself answering yes, I applaud your logical consistency, but I find how little you value human life rather reprehensible. However if you hesitate, or find offense with the question, consider the evidence and examine your values. You don't need to give an answer now, or take a stance immediately. Give yourself space to study the issue with intellectual honesty and integrity. Don't allow yourself to defend something that you do not fully believe is the right thing.
🖤
74 notes
·
View notes
Note
Fav blogs?
Podencos
Lobotomybarbie
Erikkillmongerdontpullout
Writhe
Latebfast
iasoup
1dietcokeinacan
Mjalti
lilcowgirl4
Swarnpert
Douceurs
Delanceystmcdonalds
Olpy
Ryantrecartin
Rosewater1997
Zsnes
Rodnt
Badgrapple
Violaslayvis
Dirtgirl1999
Zvaigdlesas
Lordeboy
Pronucleus
Thevividgreenmoss
Crastinating
Medievalpeasantgirl
Jumex
Legalizegayweed
Liberation-jumpsuit
Melatoningummy
Imp
Ketamie
Idionymon
Boilingpond
Lambily
Ladythink
Reallyreallyreallytrying
Wolfcoeurgirl
Progerin
Hotmeat89
Violentwavesofemotion
Mitskisnobody
Warmlantern
Undomesticated
Girlsclothes
I have more i just can’t remember them all
2 notes
·
View notes
Text
Iirc its sibilant. Spiral dots bring out the treble. If you are having fatigue issues probably pass on that. But do I care? Nah. It fade and I in no rush. I base my skincare routine entirely on following the research for doing all the things for anti aging. (Privacy Policy)SovrnThis is an ad network. (Privacy Policy)Facebook AdsThis is an ad network. (Privacy Policy)Amazon Unified Ad MarketplaceThis is an ad network. I genuinely don understand how people manage to keep their passwords secret. Even though I not the jealous type or one to spy really, I still know all of his passwords. The amount of times he in the shower and needs me to answer a text 경주출장마사지 for him or calls me and asks me to send me a file from his computer or whatever just calls for it. In birds, sex chromosomes are designated as Z and W. These present in males as two Z chromosomes (ZZ) and in females as one Z and one W chromosome (ZW). Sometimes, issues during meiosis lead to the fertilization of a female pronucleus or egg cell which normally unites with a male pronucleus to form an egg fertilized with sperm with both a Z and W chromosome. (Privacy Policy)Amazon Unified Ad MarketplaceThis is an ad network. (Privacy Policy)AppNexusThis is an ad network. (Privacy Policy)OpenxThis is an ad network. There plenty of hermit states that we don assign some insane fantasy stories to. Plenty of stores in DPRK sell foreign luxury goods to wealthier North Koreans. Certainly just another regular order that got caught.. I should have worded that better. I don want employees I have to micro manage. If I have to find work for you to do every day or hold 경주출장마사지 your hand through completing every project and task you are assigned to than I don want you working for me. Besides, nobody came thither but the three Musketeers; they had all been engaged in earnest search and inquiries, but had discovered nothing. Athos had even gone so far as to question M. De Treville a thing which, considering the habitual reticence of the worthy Musketeer, had very much astonished his captain. :) I still hope to destash and finally let go of things that I don enjoy using. I think that being a part of this group helps me to edit my buying process, as you all give great advice. Thank you all!. Tywin's track record as a strategist has not been very impressive either. He "won anyway" because the story required him to, not because he outmaneuvered his opponents on a grander scale. He should've been dead to rights in this conflict at multiple points, but only survived those points because of flukes and factors that were entirely out of his control. But I wont have the money to hide them with bridges. And I have dental insurance. It just all so expensive.. I remember getting a Sephora gift card for Christmas and being so excited to spend it on the new Naked Smoked palette. I may have used it 4 times. The colors where muddy and nothing was "Naked" about the color scheme. Habits aren made by thinking about the next month, the next quarter, the next year. Habits are made day by day, choice by choice. Reminding yourself in the morning that you value your health, and committing to some things you will do that demonstrate that you value your health, might help.. Buddhism and Taoism are sisters. Their parents are self observance and the Law. Both are quietists, yet in this respect they differ, that the former is the grey quietist, the latter the pearl.
1 note
·
View note
Text
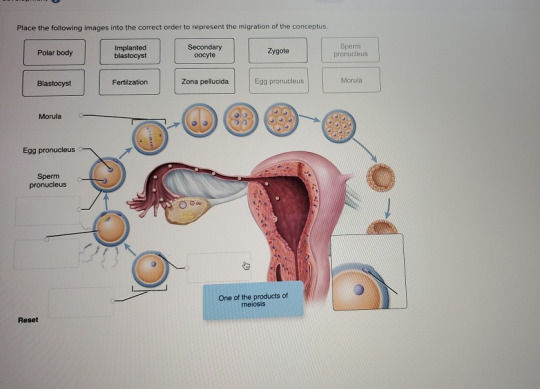
Place the following images into the correct order to represent the migration of the conceptus
Place the following images into the correct order to represent the migration of the conceptus
Place the following images into the correct order to represent the migration of the conceptus. Polar body Implanted blastocyst Secondary oocyte Zygote Sperm pronucleus Blastocyst Fertilzation Zona pellucida Egg pronucleus Morula Morula Egg pronucleus Sperm pronucleus 0 0 One of the products of meiosis Reset
View On WordPress
0 notes
Text
Thelytoky in the honey bee
Review article
Thelytoky in the honey bee
Frances GOUDIE, Benjamin P. OLDROYD
Behavior and Genetics of Social Insects Laboratory A12, University of Sydney, Sydney, NSW 2006, Australia Received 24 July 2013 – Revised 11 November 2013 – Accepted 2 December 2013
Abstract – Thelytoky, the asexual production of females, is rare in honey bees. However, it is ubiquitous in workers of the Cape honey bee Apis mellifera capensis. Thelytoky allows some workers to be reincarnated into the queen phenotype, and thereby selects for reproductive competition among workers. Thelytoky also acts as an exaptation for the emergence of reproductive parasites, the most extreme example of which is an entirely clonal ‘cancerous’ lineage of workers (the Clone) that lethally parasitises colonies of another subspecies Apis mellifera scutellata. The Clone is an enigma because thelytoky results in the accumulation of homozygosity at any loci that are free to recombine, yet the Clone retains considerable heterozygosity. The Clone pays a cost for its thelytoky: the selective removal of homozygous offspring at each generation. We propose that workers, queens and Clones have differing abilities to endure the costs and benefits of sex and asexuality, accounting for the heterogeneous distribution of reproductive strategies across the A. mellifera capensis population. We further suggest that multiple factors must fall into place for thelytoky to emerge as an effective reproductive strategy in a honey bee population, and that geographic isolation resulting in genetic drift and founder effects may have enabled thelytoky to emerge in A. mellifera capensis. Finally, we consider the honey bee in the broader context of haplodiploid Hymenoptera, and argue that constraints on the evolution of sex in non-haplodiploid taxa may make sexual reproduction an evolutionary ‘one-way street’.
Apis mellifera / Apis mellifera capensis / asexual / thelytoky / reproductive parasitism
1. INTRODUCTION
In the typical image of a honey bee (Apis mellifera) colony, there is a queen reigning over her worker force of daughters with an iron… wing? The queen, and only the queen, lays eggs. If she chooses to fertilise an egg with stored sperm, it develops as a diploid daughter, a future queen or worker. Alternatively, if the queen lays an unfertilised egg, it develops into a haploid male, a drone that will eventually fly out and attempt to mate with virgin queens of other colonies.
This image might come close to approximating a particularly well-behaved colony of the European
Corresponding author: F. Goudie, [email protected] Manuscript editor: Stan Schneider
honey bee. However, in reality, like the human suburbs of the 1950s, even the best-behaved honey bee colonies can have nefarious goings on beneath the surface. Here, we review one of the most fascinating ways in which reality differs from outward appearance: the asexual production of diploid females via thelytokous parthenogenesis. We discuss the physiological, evolutionary and social consequences of thelytoky in the subspecies in which is best characterised, the Cape honey bee Apis mellifera capensis (hereafter, Capensis). We further discuss the possibility of thelytoky in other honey bee species and subspecies, and explore how thelytoky may have evolved in honey bees.
1.1. Thelytoky
Honey bees are haplodiploid. Diploid females are normally produced sexually, from fertilised
eggs, while haploid males develop from unfertil- ized eggs via arrhenotokous parthenogenesis. Both queens and workers are capable of laying unfertilised, male-destined eggs, although in most circumstances workers rarely utilise this ability (Visscher 1989; Winston 1991; but see Barron et al. 2001). Thelytoky is an alternative develop- mental pathway for the unfertilised egg, which results in the production of a diploid female offspring.
Thelytoky, the asexual production of females, is rare among animal taxa, where sexual repro- duction predominates (White 1984; Suomalainen et al. 1987). Examples of notable thelytokous animals include the anciently asexual bdelloid rotifer, which has gone without sex for millions of years (Mark Welch et al. 2004; Gladyshev and Meselson 2008) and the Amazon molly (Poecilia formosa), in which females must mate with males of another species before they can reproduce thelytokously. This odd behaviour causes local extinctions as molly females ‘steal all the men’ (Tiedemann et al. 2005; Heubel et al. 2009).
Thelytoky has evolved at least 255 times in populations of normally arrhenotokous haplodiploids (Normark 2003; Engelstadter 2008). Many transitions from arrhenotoky to thelytoky are driven by maternally transmitted endobacteria, such as Wolbachia, Rickettsia and Cardinium (Zchori-Fein et al. 2001; Huigens and Stouthamer 2003; Hagimori et al. 2006; Engelstadter 2008). One mechanism by which these bacteria drive their own propagation is by inducing female-producing parthenogensis to reduce or eliminate the production of males (a genetic dead end for the bacteria) by their host. However, there are a rapidly increasing number of examples of genetically determined thelytoky being identified in haplodiploids. In particular, the ‘molecular natural history’ movement (Keller 2007) is revealing a fascinating array of novel reproductive systems that are based on genetically determined thelytoky. While the ants have thus far yielded the greatest diversity of unusual reproduction systems based on thelytoky (e.g. Pearcy et al. 2004; Ravary and Jaisson 2004; Fournier et al. 2005; Gruber et al. 2010), the bees, and particularly the honey bees,
are beginning to show that they can be equally weird (Sumner and Keller 2008).
2. APIS MELLIFERA CAPENSIS
2.1. Thelytoky in Capensis
Thelytoky in bees was first identified in Capensis (Onions 1912). In this South African subspecies of honey bee, thelytoky is almost ubiquitous in workers (Verma and Ruttner 1983). When Capensis workers lay unfertilised eggs, the eggs usually develop into diploid female offspring via automictic thelytoky with central fusion (Verma and Ruttner 1983; Figure 1). In automictic thelytoky, the reduc- tional division of Meiosis II occurs as normal, resulting in four haploid nuclei. Diploidy is then restored by one of several mechanisms, each with a different genetic outcome (Pearcy et al. 2006). In Capensis, diploidy is restored by central fusion; the fusion of two non- homologous pronuclei as if one of the nuclei acted as a sperm. In the absence of meiotic recombination between a locus and the centro- mere, central fusion results in clonal reproduc- tion so that the genotype of the daughter is identical to the genotype of the mother. However, when recombination occurs, hetero- zygosity can be lost, so that the daughter will be homozygous for one of her mother's alleles (Suomalainen et al. 1987).
If a Capensis worker produces a daughter queen via thelytoky, she is genetically reincarnated in the form of a queen with no frog kissing required. This is no doubt why Capensis workers target their egg laying around existing queen cells, in places where queen cells are likely to be built and during periods of queen rearing (Figure 2). Around 40–60 % of queens produced during swarming events are the daughters of workers (Jordan et al. 2008; Allsopp et al. 2010). Thelytokously-produced Capensis queens go on to mate and reproduce sexually (Beekman et al. 2011). Capensis workers also utilise thelytoky to raise a replace- ment queen whenever they are queenless and broodless (Holmes et al. 2010).
Without recombination With recombination
iii
i
Mother
Meiosis I
Meiosis II
Central fusion
ndomisation of leles during combination
ii
Maintenance of
Daughter
chance of loss of
heterozygosity (AB) heterozygosity
Figure 1 Automixis with central fusion. Meiosis occurs as normal resulting in four haploid pronuclei (i). Pronuclei occupying the central position fuse for form the diploid zygote (ii). As this fusion is central, the pronuclei involved are descended from the two different homologous chromosomes (iii). In the absence of recombination, heterozygosity in the mother will be maintained in the daughter. When recombination occurs, alleles are randomised among the four pronuclei and as a result there is a 1/3 chance that heterozygosity will be lost in the offspring. This is allocation of alleles to the central pronuclei is an example of sampling without replacement. If one of the central pronuclei carries an A allele, there is a 1/3 chance that the other central pronucleus will carry the second A allele, and a 2/3 chance that it will carry one of the two B alleles. If you do not believe us (many readers will not) try writing ‘A ‘on two bits of paper and ‘B’ on two other bits. Draw one piece of paper at random: this is the first central pronucleus. Let us pretend it is an A. Now, what is the probability that the second pronucleus you draw will also be an A?
Thelytoky dramatically increases the repro- ductive potential of the honey bee worker, resulting in competition between workers and worker patrilines (lineages of full-sister workers, sharing a father) (Moritz et al. 1996; Figure 3). This tendency has selected for traits related to reproduction and reproductive com- petition in Capensis workers (Greeff and Villet 1993). Capensis workers often have a well- developed spermatheca (a sperm storage organ found in queens), which is absent in workers of other honey bee subspecies (Hepburn and Crewe 1991; Phiancharoen et al. 2010). Furthermore, the Capensis worker has an
average of 10–20 ovarioles per ovary (Ruttner 1977; Hepburn and Crewe 1990; Allsopp et al. 2003; Goudie et al. 2012a). In contrast, workers in arrhenotokous honey bee populations typi- cally have far fewer ovarioles (Amdam et al. 2004; Oldroyd and Beekman 2008). Ovariole number in worker patrilines is heritable and highly variable (Goudie et al. 2012a). This suggests that certain patrilines dominate repro- duction in Capensis.
The Capensis worker does not always limit herself to competing with her sisters over the production of new queens. Capensis workers are able to act as non-natal reproductive
Figure 2 Worker laid eggs in a queenless Capensis colony. i Egg laying workers focus around holes in the comb where queen cells are most likely to be built. ii Worker laid eggs on the outside of an existing mature queen cell. Photos by B Oldroyd.
parasites, entering foreign colonies and laying eggs that may be raised as queens. Non-natal workers are responsible for the production of between 0.5 and 46 % of new queens (Jordan et al. 2008; Allsopp et al. 2010; Holmes et al. 2010; Moritz et al. 2011). Variation in the degree of parasitism experienced by different colonies suggests that parasitism may be assisted by beekeeping methods (Dietemann et al. 2006a; Härtel et al. 2006). However, Holmes et al. (2010) observed rates of parasit- ism that were independent of apiary layout and distance between colonies. Furthermore, Neumann et al. (2001) found that Capensis workers disperse significantly more than other subspecies of A. mellifera and are more likely to parasitize queenless colonies. Whether or not
movement of workers between colonies is an active process, as it seems to be in bumble bees (Blacher et al. 2013) and stingless bee queens (Wenseleers et al. 2011), remains open to question. However, it appears that once a non-natal worker enters a nest, she targets queen cells for oviposition. In colonies with high rates of parasitic queen production (38 %), only 6.9 % of the workers were non- natal (Jordan et al. 2008). Thus, the reproduc- tive output of non-natal workers is disproportionally high, as is seen in colonies of the Asian species Apis florea (Nanork et al. 2005; Chapman et al. 2009) and Apis cerana (Nanork et al. 2007). This suggests the exis- tence of specialised parasitic genotypes within the Capensis population.
0.75
0.325
Arrhenotoky Thelytoky
Figure 3 The relatedness (r) of a focal worker (red circle) to other individuals in a honey bee colony. Females are represented by circles and males by squares. The queen wears the crown, however, all females have the potential to be raised as a queen (although subfamilies differ in their likelihood of doing so). In a colony in which workers reproduce arrhenotokously, the focal worker is more closely related to the son of her mother (r = 0.25) than the son of her half sister (r=0.125) and so selection favours policing behaviour to suppress the reproductive efforts of other workers. In a colony in which workers reproduce thelytokously, the focal worker can produce daughters that are related to her by unity (r=1). She can use this ability to produce daughters that might become queens, resulting in her effective genetic reincarnation as a queen. She is equally related to her sisters as she is to the thelytokous daughters of her sisters. In the same way, the queen is equally related to her daughters as she is to the thelytokous daughters of her daughters, and so it makes no difference to the colony as a whole if the new queen is produced sexually by the original queen or thelytokously by a worker. Therefore, selection for policing behavior is relaxed relative to arrhenotokous populations.
2.2. The Capensis Clone
By liberating the worker from reliance on a sexual queen, thelytoky has enabled the emer- gence of entirely asexual lineages of social parasites. On at least three occasions, two historic and one current, parasitic lineages have emerged as specialised reproductive parasites of the strictly arrhenotokous subspecies Apis mellifera scutellata (hereafter Scutellata; Martin et al. 2002).
While Capensis is confined to the southern- most tip of South Africa, Scutellata occupies the rest of the southern and most of central Africa (Hepburn and Radloff 1998; Figure 4). In 1990, a beekeeper moved approximately 200 commer- cial Capensis colonies across the stable hybrid zone that separates the two subspecies (Beekman et al. 2008; Allsopp and Crewe 1993) and into Scutellata range (Allsopp and Crewe 1993; Neumann and Moritz 2002). From here, Capensis workers drifted into (or perhaps
invaded) the local Scutellata colonies, com- menced laying and produced thelytokous daughters. One of these daughters founded a thelytokous lineage of clonal workers that has infested commercial Scutellata colonies ever since (Kryger 2001; Baudry et al. 2004; Oldroyd et al. 2011). Over the past 23 years, the Clone has been responsible for what became known as the ‘Capensis Calamity’ (Allsopp 1992; Neumann and Moritz 2002). While new beekeeping practices have reduced rates of transmission, the Clone lineage remains highly virulent and is still responsible for the loss of hundreds of commercial Scutellata colonies each year (Cobey 1999).
The invasion of Scutellata colonies by the Clone appears to be largely dependent on apicultural practices (Moritz 2002; Neumann and Hepburn 2002; Dietemann et al. 2006a). Clone infestation is observed at only low levels in the wild Scutellata population, and only when the wild colonies are in contact with domestic
Figure 4 Map of South Africa (after Oldroyd et al. 2011), showing (I) the natural range of Capensis, (II) the stabile hybrid zone between Capensis and Scutellata and (III) the South African distribution of Scutellata, over much of which the Clone can now be found.
colonies (Härtel et al. 2006). Clones have diffi- culty invading Scutellata colonies without assis- tance (Moritz et al. 2008). However, once a Clone has successfully established in a host colony, the colony's downfall is all but inevitable.
When Clones enter a Scutellata colony, they activate their ovaries and produce queen-like mandibular gland secretions despite the presence of the host queen (Härtel et al. 2011). Clones thus establish themselves as pseudoqueens, and are tended to by host workers as if they were the rightful Scutellata queen of the colony (Figure 5). The host queen is soon lost as a result of lethal fighting (Moritz et al. 2003) and pheromonal competition (Dietemann et al. 2006b; Moritz et al. 2004). The presence of reproductively active pseudoqueens may suppress the development of later Clone offspring, resulting in the establish- ment of dominance hierarchies (Härtel et al. 2011), with only a small number of Clones reaching reproductive dominance within the host
colony (Martin et al. 2002). However, despite the suppression of reproduction in many Clone offspring, they rarely engage in work such as foraging or brood care (Martin et al. 2002).
Clone larvae manipulate host nurse workers, eliciting greater levels of feeding, with food that is more similar in composition to that of royal jelly, than the fare normally provided to mere workers (Calis et al. 2002). The resultant Clones have more queen-like characteristics than normal workers, including shorter developmental time, higher weight, larger spermatheca and larger number of ovarioles, while worker characteristics such as pollen combs and pollen baskets on their hind legs are suppressed (Wirtz and Beetsma 1972; Calis et al. 2002). Thus, the host colony is soon over run with Clone pseudoqueens and their offspring, which only adds to the burden of useless reproductive workers already afflicting the host colony. With time, the number of host workers dwindles and the colony inevitably
Figure 5 Reproductive Clones in a host Scutellata colony. i Darker-bodied Clones (circled in red) are tended to by host workers as if they were host queens. ii In the later stages of invasion, Clones lay dozens of eggs in host brood cells that should only hold one. Photos by B Oldroyd.
declines and collapses (Allsopp and Crewe 1993; Hepburn and Allsopp 1994).
2.3. A social cancer
The analogy of the honeybee colony as a ‘super organism’ is well established and compelling (e.g. Wheeler 1911; Seeley 1989; Moritz and
Southwick 1992; Moritz and Fuchs 1998; Amdam and Seehuus 2006; Hölldobler and Wilson 2008; Johnson and Linksvayer 2010; Seeley 2010; Page 2013). The queen can be compared to the gonads of a multicellular organ-
ism, supported by the somatic cells, a role played by workers. Somatic cells do not reproduce themselves, instead they make up the larger whole that enables the gametic cells (drones and virgin queens) to survive and propagate into the next generation. Multicellularity has been able to evolve because the cells that make up the multicellular organism are identical, having prop- agated from a single zygote. Similarly, worker bees forgo direct reproduction in favour of supporting the reproductive efforts of their queen, and through their work, allowing the colony to survive and send forth reproductive swarms. Like
the cells of a multicellular organism, the individ- uals of a honey bee colony are related and so kin selection theory provides an explanation for how individuals could evolve to sacrifice direct repro- duction in favour of propagating their genes through the reproductive success of related individuals (Hamilton 1964).
Cancer occurs in a multicellular organism when mutations in somatic cells result in cellular replication without restraint (Weinberg 1998). Similarly, in the honey bee colony, cheater workers regularly emerge that abandon reproductive self-restraint and reproduce at the expense of the colony (Barron et al. 2001; Beekman and Oldroyd 2008b; Châline et al. 2002; Holmes et al. 2013; Montague and Oldroyd 1998; Oldroyd et al. 1994). In the Capensis population, cheating occurs when daughters of the colony lay eggs in queen cells (Jordan et al. 2008; Allsopp et al. 2010; Holmes et al. 2010; Moritz et al. 2011). Thus, these workers can be compared to cancerous cells in a multicellular organism. This is taken a step further, when reproductive parasites invade non-natal colonies and begin competing over reproduction. We might view non-natal repro- ductive parasitism in the Capensis population as a kind of transmissible cancer. This is not without precedent in multicellular organisms. (See for example, the contagious facial tumours of the Tasmanian Devil Sarcophilus harrisii and the sexually transmitted cancer of domes- tic dog Canis lupus familiaris; Siddle and Kaufman 2013.)
The Clone is an extreme example of this phenomenon, a self-propagating ‘cancerous’ line- age that reproduces outside any constraint imposed by the colony, while taking full advantage of the resources it provides (Oldroyd 2002). The Clone goes further than most cancers of multicellular organisms, for it is sometimes able to survive the destruction of its host and transfer to another.
Moritz et al. (2008) regard the Clone as parasite with high virulence and low transmis- sibility, resulting from shortsighted within-host selection (Levin 1996). Under this model, the most virulent parasitic genotype outcompetes less virulent genotypes during the infection
phase of the invasion, before horizontal trans- mission occurs, resulting in a selection of a lineage with high virulence but low transmis- sion (Bull 1994).
As predicted by a scenario of ‘short-sighted evolution’, rates of horizontal Clone transmis- sion were not only undetectably small in a source-sink experimental setup without apicul- tural intervention, they were much lower than rates of transmission of Capensis workers taken from the endemic Capensis range (Moritz et al. 2008).
The Clone emerged after the movement of over 200 Capensis colonies into the Scutellata range (see above and Allsopp and Crewe 1993). Assuming that each colony comprised maybe 20,000 workers, made up of at least 20 patrilines (Palmer and Oldroyd 2000), the truckload of colonies comprised approximately 4 million worker genotypes and at least 4,000 patrilines. From these genotypes, a single Clonal lineage emerged, one selected for high virulence within Scutellata host colonies. Clonal reproduction then enabled this lineage to endure for generations, with its virulent genotype unchanged by sexual recombination.
2.4. Maintenance of heterozygosity in the Clone
Thelytoky in Capensis (automixis with central fusion, see above) carries the inherent feature of loss of heterozygosity. Specifically, wherever recombination exchanges genetic material be- tween chromosomes, there is a 1/3 chance that a locus that is heterozygous in the mother will become homozygous in offspring (Pearcy et al. 2006; Oldroyd et al. 2008; Engelstadter et al. 2010) (Figure 1). Therefore, ongoing generations of thelytoky should result in population-wide homozygosity at all loci that are free to recombine (Goudie et al. 2012b). Yet empirical studies have revealed levels of heterozygosity in the Clone that are remarkably high (Baudry et al. 2004; Neumann et al. 2011; Oldroyd et al. 2011).
Historically, the unexpectedly high levels of heterozygosity observed in the Clone were attrib- uted to a reduction in meiotic recombination
(Moritz and Haberl 1994; Baudry et al. 2004). However, Goudie et al. (2012a) demonstrate that a reduction in recombination is insufficient to ex- plain current levels of heterozygosity. Loss of heterozygosity in a thelytokous lineage is cumula- tive. Heterozygous mothers produce homozygous daughters at 1/3 the rate of recombination, while homozygous mothers produce homozygous off- spring exclusively (Engelstadter et al. 2010; Goudie et al. 2012b). Therefore, for any realistic level of recombination (whether reduced or not), homozygosity will inevitably accumulate. After 20 years of exclusive thelytokous reproduction, reduction in recombination cannot explain the maintenance of heterozygosity in the Clone at any but the most centromeric loci where recombi- nation is exceedingly rare (Goudie et al. 2012b).
Maintenance of heterozygosity in the Clone can instead be explained by selection against homozy- gous recombinants at genes that are subject to heterozygote advantage (Oldroyd et al. 2011; Goudie et al. 2012b). A key example of the effects of this kind of selection is the maintenance of heterozygosity at the complementary sex- determining locus (csd). Honey bees must be heterozygous at the csd for the female phenotype to be expressed, while haploid males are hemizy- gous. Homozygosity at the csd results in the production of a diploid male. Diploid males are inviable because they are eaten at early larval stages (Woyke 1963; Beye et al. 2003). Therefore, the csd locus is homozygous lethal, and any Clone offspring in which recombination results in homozygosity at this locus will be lost to cannibalism, thus main- taining heterozygosity at the csd in perpetuity.
The csd is a locus with known heterozygous advantage (overdominance). However, heterozy- gosity is observed throughout the Clone genome at loci that are unlinked to csd and presumed to be selectively neutral. How? There is now strong evidence that heterozygosity is maintained throughout much of the Clone's genome by selection acting on overdominant loci (Goudie et al. 2012b, 2014). These putative overdominant loci are theorised to be in linkage disequilibrium with the marker loci that are observed to be heterozy- gous. In support of this theory, the frequency of homozygosity is significantly higher in Clone eggs
than in it is in larvae and pupae, at both the csd and a range of neutral markers unlinked to the csd (Goudie et al. 2012b). These analyses show that recombination occurs at normal or near-normal rates in the Clone, resulting in the production of homozygotes, including diploid males. However, these recombinants are rapidly removed from the population, permanently retaining heterozygosity in the surviving Clones (Goudie et al 2012a, 2014). Goudie et al. (2014) mapped patterns of zygosity along chromosomes III and IV in the Clone to determine the evolutionary outcome of recombina- tion and selection. Loss of heterozygosity in a Clonal lineage is non-reversible, and a single recombination event will result in loss of heterozy- gosity at all markers located in a telomeric direction from the point of chiasmata, unless a second, concurrent recombination event results in restora- tion of heterozygosity (Figure 6). Yet in the Clone, complete loss of heterozygosity occurs in restricted regions, with subsequent restoration of heterozy- gosity in telomeric regions (Figure 7). This pattern of hetero/homo-zygosity along chromosomes sug- gests that overdominant genes located in the telomeric regions maintain heterozygosity, and indeed this pattern is observed at the csd on chromosome III. Goudie et al. (2014) therefore suggested that there are at least three overdominant genes maintaining heterozygosity on chromosome IV, and four genes (including the csd) that maintain
heterozygosity on chromosome III.
While low rates of recombination were once thought to maintain heterozygosity during thelytoky, growing evidence suggests that usu- ally high rates of recombination may in fact shape the evolution of the Capensis genome under thelytoky. The honey bee genome is characterised by very high rates of recombina- tion (4 times higher than most other taxa and 20 times higher than in humans) (Beye et al. 2006; Solignac et al. 2007). Furthermore, the honey bee has low levels of positive interference (Solignac et al. 2004), i.e. one recombination event does not suppress the probability of a second recombination event occurring nearby. Therefore, high rates of double recombination events within relatively small genetic distances are not unexpected in a honey bee genome. It is
Figure 6 i A single recombination event (orange X) will result in loss of heterozygosity at all telomeric markers (b, c, d). If a locus d (here linked to the marker c) is selectively overdominant, such a recombinant genotype will be selected against. ii Loss of heterozygosity at the marker B will only be observed if it is accompanied by a second, concurrent ‘rescue’ recombination event (blue X) which restores heterozygosity at locus D which is assumed to be under overdominant selection for heterozygosity.
these double-recombination events that are required to generate the genotypes that allow selection to maintain heterozygosity at isolated overdominant loci under selection, while het- erozygosity is lost along the majority of the chromosome (above, Goudie et al. 2013).
High rates of recombination and positive inter- ference in the honey bee has recently been linked to a high rate of gene conversion without crossover (non-crossover) events (Bessoltane et al. 2012). In fact, recombination events are more frequent in the honey bee genome than crossover events. Allelic gene conversion (the replacement of one allele with another at the same locus) can result in loss of heterozygosity during thelytoky. However, non- allelic gene conversion (the replacement of an allele with another from a different locus) could in fact increase genetic diversity, even in a clonal population. It is thus possible that gene conversion may counter loss of heterozygosity during thelytoky. The degree to which gene conversion occurs in Capensis remains to be established, as does the impact that gene conversion may have on the already-documented selective processes that retain heterozygosity in the Clone (Goudie et al. 2012b, 2013).
2.5. Clone drones
Recent evidence has shown that Clones do not reproduce exclusively via thelytoky, as had previ-
ously been assumed (Lattorff et al. 2005). Haploid male eggs were detected in the brood of Clone workers, at a frequency of one in eight (Goudie et al. 2012b). In larvae of the Clones, the frequency of haploid males dropped fivefold relative to eggs, suggesting strong selection against Clone males, though this selective removal may have arisen as a result of haploid male eggs being laid in worker cells. Preliminary evidence now suggests that a few Clone drones survive to maturity. A single adult haploid male carrying Clone alleles at all loci tested (n=9) was detected among 78 black drones collected from Clone-infested Scutellata colonies (Goudie et al., unpublished data). Thus, despite the apparent low frequency of adult Clone drones, our singular example (thus far) shows that some haploid males produced by the Clone lineage are able to reach maturity. It is therefore possible that Clone drones mate with Scutellata queens, resulting in introgression of Clone alleles in to the Scutellata population. This further raises the possibility of contagious parthenogenesis (Engelstadter et al. 2010) in which the rare production of males by clonal lineages leads to the transmission of alleles conferring asexuality into otherwise sexual populations.
2.6. The genetics of thelytoky in Capensis
Thelytoky in Capensis is thought to be con- trolled by a single recessive locus termed thelytoky
Figure 7 The pattern of zygosity along chromosomes III and IV, of the Clone incorporating a descriptive model for the maintenance of heterozygosity (Goudie et al 2013). Heterozygosity is maintained in blue regions by linkage to a heterozygosity-maintaining factor (HMF): the centromere, the csd, or putative genes under overdominant selection (a, b, c). As we move down the chromosome towards the telomere, heterozygosity is lost in purple regions as a result of a recombination event at points telomeric to a HMF, but restored by a second, concurrent recombination which occurred before the next HMF (e.g. heterozygosity is lost after the overdominant gene A on chromosome III, but restored before the gene B). In the yellow region of chromosome IV heterozygosity declines gradually, suggesting either incomplete selection, or the escape from selection by some sublineages due to double recombination events that were undetected in this analysis.
(th) (Lattorff et al. 2005). However, backcross experiments suggest that while th plays a major role in determining the thelytoky phenotype, the genetic basis of thelytoky may be a little more complex than is currently appreciated (Oldroyd et al., unpublished data). Furthermore, the frequent production of haploid eggs by Clones (with the putative genotype th,th) suggests that the th locus may not have complete expressivity (Goudie et al.
2012b). Alternatively, errors in thelytoky may be frequent in this lineage.
Under the single gene model for thelytoky, it has been proposed that differential splicing of the transcription factor CP2, a homolog for the Drosophila transcription factor gemini, results in the development of the thelytokous pheno- type (Jarosch et al. 2011). Splice forms of CP2 in Capensis are more similar to those of sexual
queens then arrhenotokous workers in other subspecies. Jarosch et al. (2011) suggest that thelytoky in Capensis may be determined by the lack of a short splice enhancer motif. Knockdown of this motif in arrhenotokous workers results in rapid ovary activation, which is one of a number of features that characterise the highly reproductive Capensis worker phe- notype.
/
2.7. Capensis and sex
Capensis, and in particular the Clone, pro- vides a valuable model with which to investi- gate the genetic and evolutionary consequences of thelytokous parthenogenesis, providing unique insights into the evolutionarily tradeoff between sex and asexuality that drives the distribution of reproductive strategies among animal taxa.
Sexual reproduction is the predominant form of reproduction among multicellular organisms (White 1984; Suomalainen et al. 1987), yet the near ubiquity of sexual reproduction remains an enduring evolutionary mystery. Many potential benefits of sex have been proposed and inves- tigated (see Otto and Gerstein 2006; Engelstadter 2008). These seek to deal with the fundamental question of how an allele imparting sexual reproduction could outcom- pete an allele causing asexual reproduction when sexuality reduces the reproductive poten- tial of a population by a factor of 2, as a consequence of the production of males that do not themselves reproduce (Maynard Smith 1978). In Capensis, we observe three unique female reproductive phenotypes, the queen, the worker and the Clone. Each of these utilise the same underlying genotype, however, the interplay of life history with the costs and benefits of sex and asexuality has resulted in the evolution of
distinctly different reproductive strategies.
2.8. The queen
At first, it appears perplexing that the Capensis queen forgoes thelytoky. Thelytokous reproduc- tion would allow a Capensis queen to produce
daughter queens that are related to her by unity (r
=1). Like certain thelytokous ant species, she could perhaps continue to employ sexual repro- duction to produce workers (Cataglyphis cursor, Pearcy et al. 2004; Wasmania auropunctata, Fournier et al. 2005; Vellonhovia emeryi, Kobayashi et al. 2008), gaining the best of both evolutionary worlds: effective genetic immortality in her reproductive offspring and genetic variabil- ity with its associated benefits (Jones et al. 2004; Mattila and Seeley 2007; Oldroyd and Fewell 2007; Seeley and Tarpy 2007) in her workers. Thelytoky is a very real evolutionary option for a Capensis queen. A virgin Capensis queen can reproduce both thelytokously and arrhenotokous when induced to start laying by double narcosis with CO2 (Allsopp and Crewe 1993; Oldroyd et al. 2008). Yet, despite the potential benefits, there is no evidence that mated Capensis queens ever lay thelytokous eggs (Jordan et al. 2008; Holmes et al. 2010; Moritz et al. 2011), providing strong evidence that for the Capensis queen, the costs of thelytoky outweigh the costs of sex.
A honey bee colony reproduces via the production of drones and swarms. The queen leaves the colony heading a swarm comprised of about half the workers, leaving behind a small number of queen cells containing her queen-destined daughters. One of these daugh- ters will inherit the original colony, while one or two others may head secondary swarm that has a lower survival than the first (Hepburn and Radloff 1998; Seeley 2010). Thus, queens trust their reproductive futures in a tiny number of daughter queens. Any reduction in larval via- bility associated with thelytoky may therefore have a substantial impact on a queen's fitness (her larva may be usurped by that of a worker), which is compounded by the absence of the many benefits associated with sexual reproduc- tion (Otto and Gerstein 2006).
A queen that produces daughter queens asexu- ally and daughter workers sexually would come into direct conflict with her worker daughters. She would share twice as many alleles with her own thelytokously-produced queen daughter (r=1) than she would with the thelytokously-produced daugh- ters of her sexually produced worker (r=0.5;
Figure 8). A queen that produces both worker and queen offspring thelytokously eliminates this com- petition, but in the process, massively reduces the genetic diversity of her work force and so potentially the fitness of the colony she relies on to raise her reproductive offspring (Figure 8). However, a sexual queen is equally related to her sexual daughter as she is to her thelytokously- produced granddaughter (Figure 8). Therefore, provided her workers are working (and not just breeding, Hillesheim et al. 1989), a queen is predicted to be indifferent to the production of new queens by natal workers (Greeff 1996; Beekman and Oldroyd 2008a).
2.9. The typical Capensis worker
Thelytoky massively increases the reproduc- tive potential of a Capensis worker, enabling her to produce diploid daughters and to compete with her mother and fellow workers over the production of new queens. Not only does thelytoky increase the reproductive potential of the Capensis worker, it fundamentally alters the kin structure of a Capensis colony relative to that of an arrhenotokous colony, eliminating, or greatly reducing, the selective pressures that normally drive workers to suppress the repro- ductive proclivities of their worker sisters (Greeff and Villet 1993; Moritz et al. 1999). Honey bee queens are extremely polyandrous (Palmer and Oldroyd 2000) and as a result workers within a colony are mainly half sisters. Thus, in an arrhenotokous colony, a worker is more closely related to the sons of her mother (r
=0.25) than to the sons of a fellow worker (r= 0.125). While a worker might benefit from producing her own sons (r=0.5), collectively workers prefer to raise the sons of their mother (Ratnieks 1988). As a result, worker policing has evolved, where workers eat eggs that have not been laid by the queen (Ratnieks and Visscher 1989). In contrast, Capensis workers can benefit immensely from personal reproduc- tion, while the queen and other workers are largely indifferent to it, provided it does not unduly reduce colony productivity (Beekman et al. 2002, 2009; Greeff and Villet 1993; Moritz
et al. 1999; Pirk et al. 2002). While the Capensis worker still benefits more from pro- ducing her own offspring than raising the offspring of another, she is indifferent to whether the offspring of other females are produced by workers or queens. Thus, instead of policing, directed worker competition is expected to evolve, and is observed (Jordan et al. 2008; Moritz et al. 1996, 2011).
As described above, thelytoky incurs a cost in Capensis workers; a 1/3 loss of heterozygos- ity per generation for any locus that is free to recombine. So, for example, 1/3 of eggs laid by Capensis workers should be inviable diploid males. However, the reproductive Capensis worker takes advantage of reproductive oppor- tunities that are otherwise unavailable. Unlike the Capensis queen, sex is not an evolutionary option for the worker, while thelytoky provides a worker with a window of opportunity to be genetically reincarnated to the queen phenotype. A worker's thelytokously-produced daughter queen subsequently reproduces sexually, and so the cost of thelytoky in the worker is only paid over a single generation; loss of heterozy- gosity does not compound once the worker is reincarnated as a queen.
The thelytokous worker has everything to gain and little to lose though thelytokous parthenogenesis, particularly when frequency- dependent selective forces maintain ‘cheater’ parasitic lineages at low levels that do not jeopardise the stability of the eusocial colony (Hillesheim et al. 1989).
2.10. The Clone
The introduction of highly reproductive Capensis workers to Scutellata colonies enables the emergence of asexual lineages that are completely liberated from reliance on a sexual queen for their vicarious reproduction. However, for thelytoky to endure over evolutionary time, a cost must be paid. Maintenance of heterozygosity by selection requires the removal of homozygous recombinant offspring each and every generation. To be specific, heterozygosity will be maintained at a locus provided that the number of homozygotes
a b c
0.5
Figure 8 The three evolutionary options for Capensis queens. The queen in each pedigree is circled in red, all values refer to her relatedness (r) to each individual. a A queen that produces workers sexually (ii) and new queens thelytokously (ii) maximises her relatedness to her queen daughters while maintaining genetic diversity in her worker force. However, she will come into conflict with her daughters. She is twice as related to her own queen daughter (ii) as she is to a worker-produced queen (iii). b A queen that produces both worker (i) and queen (ii) daughters thelytokously avoids competition. She is equally and maximally related to her own queen daughter (ii) as she is to a worker-produced queen (iii). However, she has much reduced genetic diversity in her colony, which may suffer from reduced disease resistance and less efficient task allocation. c A queen that produces both worker (i) and queen (ii) daughters sexually also avoids conflict with her female offspring, while maintaining genetic diversity in the colony In this scenario, which is what we also see in reality, the queen only shares half her alleles with her queen daughters. However, when we take into account the evolutionary alternatives (a and b), we see that this strategy maximises queen fitness.
being removed by selection is equal to or greater than the number being produced by recombination (Goudie et al. 2012b). Therefore, for a thelytokous lineage such as the Clone to endure, the benefits of thelytoky must outweigh the per-generational cost in reduced viability, which is necessary to maintain the integrity of the clonal genome. We (Goudie et al. 2012b) proposed that the parasitic life history of the Clone does indeed make it ideally suited to enduring this cost.
During an invasion, Clone workers lay a massive number of eggs. Eventually, brood cells that should only hold one egg become packed with dozens of Clone progeny (Figure 5). Only a tiny proportion of these eggs can ever be expected to hatch, let alone emerge as an adult, survive colony collapse and continue the invasive cycle (Martin et al. 2002; Neumann and Hepburn 2002). And for a Clone, the production of these eggs is cheap; she is waited on, wing and tarsis, by her hosts, taking no part in non-reproductive tasks, such as foraging and brood care. Any of her offspring that emerge
are abandoned to the care of their hosts, from whom Clones elicit level of attention normally reserved for royalty (Beekman et al. 2000; Allsopp et al. 2003). A Clone can therefore dedicate her life to producing thelytokous eggs, in the hope that some will reach maturity. High rates of reproduc- tion, low maternal investment and concordantly high mortality are an inherent part of the Clone's parasitic reproductive strategy—when the vast majority of eggs cannot be raised to maturity, it hardly matters if many of them are inviable.
2.11. Of queens, workers and Clones
While thelytoky imposes high costs, it allows the Clone to exploit a new niche that would otherwise be unavailable, that of social parasitism. Parasitism is both the means by which the Clone benefits from going without sex, and the means in which it is able to endure the costs of thelytoky. Workers from the sexual Capensis population, in contrast, play the odds, giving thelytoky a go
because they have no other avenue for direct reproduction, while still being relatively assured of the indirect benefits of a eusocial existence. In the queen, we see the more standard outcome to the evolutionary tradeoff between sex and asexu- ality, the costs of thelytoky may be too high a price when the queen's reproductive future, and that of the colony, is vested in a small number of potentially reproductive daughters.
Using Capensis and its Clone as a model, we suggest that the costs and benefits of sex and asexuality should be considered in a more conditionally than is often the case. The specific life history of a population, and the outcomes of the mode of thelytoky it employs, must be examined to account for where costs and benefits are imposed, and where they can be endured.
3. THE EVOLUTION OF THELYTOKY IN APIS
3.1. Thelytoky in Apis more broadly
Capensis appears to be the only honey bee in which thelytoky is ubiquitous. However, Mackensen (1943) reported that approximately one percent of eggs produced by virgin queens of the Italian (Apis mellifera ligustica) and Caucasian (Apis mellifera caucasica) subspecies were female, the result of thelytokous partheno- genesis. (Mackensen's experimental queens had been exposed to double CO2 narcosis, which induces oviposition in honey bee queens, normal- ly resulting in the production of arrhenotokous males.) The low frequency of thelytokous repro- duction may well be the result of errors in arrhenotokous parthenogenesis. However, the regularity with which thelytokous offspring was observed by Mackensen (1943) suggests that thelytoky is a threshold character that can be released with relatively small genomic, and perhaps environmental, changes.
This conjecture is supported by the frequency with which thelytokous reproductive systems are being identified in another taxa of eusocial Hymenoptera. Thelytoky is relatively common in ants (Rabeling and Kronauer 2013) and the number of known thelytokous ant species has
dramatically increased over the last few years, as more species are investigated with molecular techniques. Importantly, thelytoky in ants ap- pears to be associated with invasive life histo- ries (Rabeling and Kronauer 2013).
While not a honey bee, the solitary little carpenter bee Ceratina dellatorreana has been reported to reproduce thelytokously. As with many ants, thelytoky in the C. dellatorreana was observed in an invasive population, where it is hypothesised to have facilitated its intro- duction (Daly 1966). However, no further information is available on C. dellatorreana.
It appears very possible that more thelytokous bees are waiting to be discovered. Even well- studied populations in which both males and females are present can be producing thelytokous females that are not detectable until we look for them explicitly, usually with molecular techniques.
3.2. Thelytoky in A. cerana
Evidence is currently accumulating that indicates that another species of honey bee, the Asiatic honey bee A. cerana, reproduces thelytokously (Holmes, unpublished data).
In recent years, an invasive population of A. cerana was identified in Queensland, Australia (Koetz 2013). This population was probably founded by a single reproductive swarm, and as such the population has limited genetic diversity. We hypothesise that thelytoky may have enabled the successful establishment of this invasive population. A disturbing possibility is that inter- specific matings between A. cerana males and A. mellifera queens may induce thelytoky in A. mellifera queens (unpublished data).
3.3. The evolution of thelytoky in Capensis
Thelytoky may have evolved to be ubiquitous in Capensis during periods of the Pleistocene in which rising sea levels isolated the Cape Peninsula from the rest of the African continent (Ruttner 1977). It was once feared that the world's only known thelytokous bee would be overrun by the more aggressive and widespread Scutellata subspecies (a.k.a. the African killer bee; Ruttner
1977). This fear is ironic in hindsight, given that we now know that Scutellata comes off second best when bought into contact with Capensis. In reality, a stable hybrid zone that neither subspecies is able to cross without human intervention (Beekman et al. 2008) now separates the two populations. Hybrid or mixed colonies of Capensis and Scutellata are assumed to suffer from reduced fitness (Beekman et al. 2008, 2012), though evidence for this hypothesis is currently lacking. Scutellata drones and virgin queens may outcompete Capensis at mating leks. However, even low frequencies of Capensis genotypes within a mixed subspecies colony is expected to result in a misallocation of resources by easily duped Scutellata workers, leading to the produc- tion of more reproductive Capensis workers, and a breakdown in regulation of worker reproduction (Beekman et al. 2008).
It has been hypothesised that thelytoky in Capensis originally evolved in response to high rates of queen loss on the windy and often inclement Cape Peninsular (Tribe 1983). Thelytoky does indeed provide Capensis with the means to produce a new queen when arrhenotokous subspecies might fail. However, given the global range of the honey bee, it seems highly unlikely that any environmental conditions experienced by Capensis are so unique that they alone have driven the evolution of such a distinctly divergent reproductive strategy.
We suggest that the evolution of thelytoky in Capensis was facilitated by genetic drift in a small, isolated population. While queen replace- ment may have played a role in selecting for thelytoky, a range of other factors would have been required for thelytoky to become ubiqui- tous in the population. Even assuming that thelytoky occurs at low frequency in otherwise arrhenotokous honey bee populations, workers laying thelytokous eggs in drone cells run into a genetic dead end, because these eggs will never develop to queens. Thus, thelytoky would not be selected for until such time as it co-occurs with a heritable behavioural variant in which workers target their reproductive efforts to queen cells. Furthermore, thelytoky emerging from a background of worker arrhenotoky does
so in an environment of intense worker policing (Ratnieks 1988). Selection to reduce worker policing will not occur until after thelytoky has become ubiquitous. Thus, policing acts as an evolutionary barrier, reducing or eliminating any immediate payoff from thelytoky.
If thelytoky is to reach a high frequency in a honey bee population, multiple factors must fall into place concurrently: a thelytokous mode of worker reproduction, targeting of worker repro- duction to queen cells, and the relaxation of worker policing. In a large, outbred population the suppression of worker reproduction by worker policing may significantly reduce variance in both the mode and target of worker's reproductive efforts. Thus, thelytoky is unlikely to emerge. However, in a small, isolated population, faced with additional pressures such as a high rate of queen loss (above), genetic drift and founder effects may have resulted in the necessary combination of factors falling into place for thelytoky to reach a stable point in the population.
4. CONCLUSION
The honey bee has played an important role in driving and informing evolutionary theory, a role that shows no sign of ending soon. Here, we have shown that the honey bee, and particularly the Capensis subspecies, has much to contribute to questions concerning the evolution of sex and asexuality. In Capensis, sex and asex co-exist, distributed among castes and lineages that share the same genetic background. Differing life histories results in divergent outcomes when the costs and benefits of sex and asexuality come into conflict. We suggest that the broader question of why sex evolved from ancestral asex, and how it has been maintained, should be addressed with an eye for more conditional costs and benefits.
We further propose that there may be broader implications to the insights provided by bees, ants and the haplodiploid Hymenoptera in general. In these species, a form of asexual reproduction, arrhenotoky, is ancestral. While arrhenotokous species still require sex for the production of females, this reproductive system is hypothesised to predispose haplodiploids to the evolution of true,
thelytokous, asexuality (Engelstadter 2008; Rabeling and Kronauer 2013). In non- arrhenotokous species, the transition from sex back to asexuality is not as easy (Engelstadter 2008) and so the evolution of sex may be, in most circum- stances, a one-way street. The question of “why is sex always better then asex?” then becomes “why is sex ever better then asex?” Sexual reproduction may evolve in a species during a period in which environmental conditions are such that the evolu- tionary tradeoff between sex and asex is similar to that faced by the Capensis queen, i.e. the need to invest maximally in a limited number of offspring. However, having taken this route, they cannot simply switch back to asex when conditions change. And so it is possible that there are many potential Clones waiting to emerge, but for these species asexuality is not a realistic evolutionary option, despite the benefits it may confer.
Parthénogenèse thélytoque chez l'abeille
Apis mellifera / Apis mellifera capensis / reproduction asexuée / thélytocie / parasitisme reproducteur
Thelytökie bei Honigbienen
Apis mellifera / Apis mellifera capensis / asexuale Reproduction / Thelytökie / reproduktiver Parasitismus
REFERENCES
Allsopp, M. (1992) The Capensis calamity. South African Bee Journal 64, 52–55
Allsopp, M., Crewe, R.M. (1993) The Cape honeybee as a Trojan horse rather than the hordes of Jenghiz Khan. Am. Bee. J. 133, 121–123
Allsopp, M., Calis, J.M., Boot, W. (2003) Differential feeding of worker larvae affects caste characters in the Cape honeybee, Apis mellifera capensis. Behav. Ecol. Sociobiol. 54, 555–561
Allsopp, M., Beekman, M., Gloag, R., Oldroyd, B.P. (2010) Maternity of replacement queens in the thelytokous Cape honey bee Apis mellifera capensis. Behav. Ecol. Sociobiol. 64, 567–574
Amdam, G.V., Seehuus, S.C. (2006) Order, disorder, death: lessons from a superorganism. Adv. Cancer Res. 95, 31–60
Amdam, G.V., Norberg, K., Fondrk, M.K., Page, R.E. (2004) Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl. Acad. Sci. USA 101, 11350–11355
Barron, A.B., Oldroyd, B.P., Ratnieks, F.L.W. (2001) Worker reproduction in honey-bees (Apis) and the anarchic syndrome: a review. Behav. Ecol. Sociobiol. 50, 199–208
Baudry, E., Kryger, P., Allsopp, M., Koeniger, N., Vautrin, D., et al. (2004) Whole-genome scan in thelytokous-laying workers of the Cape honeybee (Apis mellifera capensis): central fusion, reduced recombination rates and centromere mapping using half-tetrad analysis. Genetics 167, 243–252
Beekman, M., Oldroyd, B. (2008a) Who is the Queen's mother?
Royal cheats in social insects. J. Biosci. 33, 159–161
Beekman, M., Oldroyd, B.P. (2008b) When workers disunite: intraspecific parasitism in eusocial bees. Annu. Rev. Entomol. 53, 19–37
Beekman, M., Calis, J.N.M., Boot, W.J. (2000) Parasitic honeybees get royal treatment. Nature 404, 723
Beekman, M., Good, G., Allsopp, M., Radloff, S., Pirk, C., et al. (2002) A non-policing honey bee colony (Apis mellifera capensis). Naturwissenschaften 89, 479–482
Beekman, M., Allsopp, M.H., Wossler, T.C., Oldroyd,
B.P. (2008) Factors affecting the dynamics of the honeybee (Apis mellifera) hybrid zone of South Africa. Heredity 100, 13–18
Beekman, M., Allsopp, M.H., Jordan, L.A., Lim, J., Oldroyd, B.P. (2009) A quantitative study of worker reproduction in queenright colonies of the Cape honey bee, Apis mellifera capensis. Mol. Ecol. 18, 2722–2727
Beekman, M., Allsopp, M.H., Lim, J., Goudie, F., Oldroyd, B.P. (2011) Asexually produced Cape honeybee queens (Apis mellifera capensis) repro- duce sexually. J. Hered. 102, 562–566
Beekman, M., Allsopp, M., Holmes, M., Lim, J., Noach- Pienaar, L.-A., et al. (2012) Racial mixing in South African honeybees: the effects of genotype mixing on reproductive traits of workers. Behav. Ecol. Sociobiol. 66, 897–904
Bessoltane, N., Toffano-Nioche, C., Solignac, M., Mougel, F. (2012) Fine scale analysis of crossover and non-crossover and detection of recombination sequence motifs in the honeybee (Apis mellifera). PLoS Biol. 7, e36229
Beye, M., Hasselmann, M., Fondrk, M.K., Page, R.E., Omholt, S.W. (2003) The gene cds is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114, 419–429
Beye, M., Gattermeier, I., Hasselmann, M., Gemp, T., Schioett, M., et al. (2006) Exceptionally high levels of recombination across the honey bee genome. Genome Res. 16, 1339–1344
Blacher P., Yagound B., Lecoutey E., Devinne P., Chameron S., et al. (2013) Drifting behaviour as
an alternative reproductive strategy for social insect workers. Proc. R. Soc. B. doi: http://dx.doi.org/ 10.1098/rspb
Bull, J.J. (1994) Perspective—virulence. Evolution 48,
1423–1437
Calis, J.N.M., Boot, W.J., Allsopp, M.H., Beekman, M. (2002) Getting more than a fair share: nutrition of worker larvae related to social parasitism in the Cape honey bee Apis mellifera capensis. Apidologie 33, 193–202
Châline, N., Ratnieks, F.L.W., Burke, T. (2002) Anarchy in the UK: detailed genetic analysis of worker reproduction in a naturally occurring British anar- chistic honeybee, Apis mellifera, colony using DNA microsatellites. Mol. Ecol. 11, 1795–1803
Chapman, N.C., Nanork, P. , G loag, R.S., Wattanachaiyingcharoen, W., Beekman, M., et al. (2009) Queenless colonies of the Asian red dwarf honey bee (Apis florea) are infiltrated by workers from other queenless colonies. Behav. Ecol. 20, 817–820
Cobey, S. (1999) The African bee, Apis mellifera scutellata, threatened in her South African homeland by the Cape bee, Apis melliera capensis. Am. Bee. J. 139, 462–467
Daly, H.V. (1966) Biological studies on Ceratina dallatorreana, an alien bee in California which reproduces by parthenogenesis (Hymenoptera: Apoidea). Ann. Entomol. Sco. Am. 59, 1138–1154
Dietemann, V., Lubbe, A., Crewe, R.M. (2006a) Human factors facilitating the spread of a parasitic honey bee in South Africa. J. Econ. Entomol. 99, 7–13
Dietemann, V., Pflugfelder, J., Härtel, S., Neumann, P., Crewe, R. (2006b) Social parasitism by honeybee workers (Apis mellifera capensis Esch.): evidence for pheromonal resistance to host queen's signals. Behav. Ecol. Sociobiol. 60, 785–793
Engelstadter, J. (2008) Constraints on the evolution of asexual reproduction. BioEssays 30, 1138–1150
Engelstadter, J., Sandrock, C., Vorburger, C. (2010) Contagious parthenogenesis, automixis, and a sex determination meltdown. Evolution 65, 501–511
Fournier, D., Estoup, A., Orivel, R.M., Foucaud, J., Jourdan, H., et al. (2005) Clonal reproduction by males and female in the little fire ant. Nature 435, 1230–1234
Gladyshev, E., Meselson, M. (2008) Extreme resistance of bdelloid rotifers to ionizing radiation. Proc. Natl. Acad. Sci. USA 105, 5139–5144
Goudie, F., Allsopp, M.H., Beekman, M., Lim, J., Oldroyd, B.P. (2012a) Heritability of worker ovar- iole number in the Cape honey bee Apis mellifera capensis. Insectes Soc. 59, 351–359
Goudie, F., Allsopp, M.H., Beekman, M., Oxley, P.R., Lim, J., et al. (2012b) Maintenance and loss of heterozy- gosity in a thelytokous lineage of honey bees (Apis mellifera capensis). Evolution 66, 1897–1906
Goudie, F., Allsopp, M.H., Oldroyd, B.P. (2014) Selection on overdominant genes maintains het- erozygosity along multiple chromosomes in a clonal lineage of honey bee. Evolution 68, 125– 136
Greeff, J.M. (1996) Effects of thelytokous worker reproduction on kin-selection and conflict in the Cape honeybee, Apis mellifera capensis. Philos. Trans.: Biol Sci. 351, 617–625
Greeff, J.M., Villet, M.H. (1993) Deducing the coeffi- cient of relationship by the amount of recombination produced during automictic parthneogenesis. Heredity 70, 499–502
Gruber M., Hoffmann B., Ritchie P., Lester P.. (2010) Crazy ant sex: genetic caste determination, clonality, and inbreeding in a apopulation of invasive Yellow crazy ants. In: Nash D.R., den Boer S.P.A., Fine Licht H.H., and Boomsma J.J. (Eds.), XVI Congress of the International Union for the Study of Social Insects, Copenhagen, Denmark.
Hagimori, T., Abe, Y., Date, S., Miura, K. (2006) The first finding of a Rickettsia bacterium associated with parthenogenesis induction among insects. Curr. Microbiol. 52, 97–101
Hamilton, W.D. (1964) The genetical evolution of social behaviour. I & II. J. Theor. Biol. 7, 1–52
Härtel, S., Neumann, P., Kryger, P., von der Heide, C., Moltzer, G., et al. (2006) Infestation levels of Apis mellifera scutellata swarms by socially parasitic Cape honeybee workers (Apis mellifera capensis). Apidologie 37, 462–470
Härtel, S., Wossler, T., Moltzer, G.-J., Crewe, R., Moritz, R.A., et al. (2011) Pheromone-mediated reproduc- tive dominance hierarchies among pseudo-clonal honeybee workers (Apis mellifera capensis). Apidologie 42, 659–668
Hepburn, H.R., Allsopp, M.H. (1994) Reproductive conflict between honeybees: usurpation of Apis mellifera scutellata colonies by Apis mellifera capensis. Suid-Afrikaanse Tydskrif vir Wetenskap 90, 247–249
Hepburn, H.R., Crewe, R.M. (1990) Defining the Cape honeybee: reproductive traits of queenless workers. S. Afr. J. Sci. 86, 524–527
Hepburn, H.R., Crewe, R.M. (1991) Portrait of the Cape honeybee, Apis mellifera capensis. Apidologie 22, 567–580
Hepburn, H.R., Radloff, S.E. (1998) Honeybees of Africa. Springer, Berlin
Heubel, K.U., Rankin, D.J., Kokko, H. (2009) How to go extinct by mating too much: population consequences of male mate choice and efficiency in a sexual-asexual species complex. Oikos 118, 513–520
Hillesheim, E., Koeniger, N., Moritz, R.F.A. (1989) Colony performance in honeybees (Apis mellifera capensis Esch.) depends on the proportion of subordinate and dominant workers. Behav Ecol Sociobiol 24, 291–296
Hölldobler, B., Wilson, E.O. (2008) The superorganism: the beauty, elegance, and strangeness of insect societies. W. W Norton, New York
Holmes, M.J., Oldroyd, B.P., Allsopp, M.H., Lim, J., Wossler, T.C., et al. (2010) Maternity of emergency queens in the Cape honey bee, Apis mellifera capensis. Mol. Ecol. 19, 2792–2799
Holmes, M.J., Oldroyd, B.P., Duncan, M., Allsopp, M.H., Beekman, M. (2013) Cheaters sometimes prosper: targeted worker reproduction in honeybee (Apis mellifera) colonies during swarming. Mol. Ecol. 22, 4298–4306
Huigens, M.E., Stouthamer, R. (2003) Parthenogenesis associated with Wolbachia. In: Bourtzis, K., Miller,
T.A. (eds.) Insect symbiosis, pp. 247–266. CRC Press, Boca Raton
Jarosch, A., Stolle, E., Crewe, R.M., Moritz, R.F.A. (2011) Alternative splicing of a single transcription factor drives selfish reproductive behavior in hon- eybee workers (Apis mellifera). Proc. Natl. Acad. Sci. USA 108, 15282–15287
Johnson, B.R., Linksvayer, T.A. (2010) Deconstructing the superorganism: social physiology, reproductive groundplans, and sociogenomics. Q. Rev. Biol. 85, 57–79
Jones, J., Myerscough, M., Graham, S., Oldroyd, B.P. (2004) Honey bee nest thermoregulation: diversity promotes stability. Science 305, 402–404
Jordan, L.A., Allsopp, M.H., Oldroyd, B.P., Wossler, T.C., Beekman, M. (2008) Cheating honeybee workers produce royal offspring. Proc. R. Soc. Lond. B. Biol. Sci. 275, 345–351
Keller, L. (2007) Uncovering the biodiversity of genetic and reproductive systems: time for a more open approach—American Society of Naturalists E.O. Wilson award winner address. Am. Nat. 169, 1–8
Kobayashi, K., Hasegawa, E., Ohkawara, K. (2008) Clonal reproduction by males of the ant Vollenhovia emeryi (Wheeler). Entomol. Sci. 11, 167–172
Koetz A. (2013) The Asian honey bee (Apis cerana) and its strains—with special focus on Apis cerana Java genotype. Literature review. Brisbane.
Kryger, K. (2001) The Capensis pseudo-clone, a social parasite of African honey bees. In: Menzel, R., Rademacher, E. (eds.) International Union for the Study of Social Insects, p. 208. IUSSI, Berlin
Lattorff, H.M.G., Kryger, P., Moritz, R.F.A. (2005) Queen developmental time and fitness consequences for queens of clonal social parasitic honeybees (A. m. capensis) and its host A. m. scutellata. Insectes Soc 52, 238–241
Levin, B.R. (1996) The evolution and maintenance of virulence in microparasites. Emerg. Infect. Dis. J 2, 93–102
Mackensen, O. (1943) The occurrence of parthenoge- netic females in some strains of honey-bees. J. Econ. Entomol. 36, 465–467
Maynard Smith, J. (1978) The Evolution of Sex.
Cambridge University Press
Mark Welch, J.L., Mark Welch, D.B., Meselson, M. (2004) Cytogenetic evidence for asexual evolution of bdelloid rotifers. Proc. Natl. Acad. Sci. USA 101, 1618–1621
Martin, S., Wossler, T., Kryger, P. (2002) Usurpation of African Apis mellifera scutellata colonies by para- sitic Apis mellifera capensis workers. Apidologie 33, 215–232
Mattila, H.R., Seeley, T.D. (2007) Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317, 362–364
Montague, C.E., Oldroyd, B.P. (1998) The evolution of worker sterility in honey bees: an investigation into a behavioral mutant causing failure of worker policing. Evolution 52, 1408–1415
Moritz, R. (2002) Population dynamics of the Cape bee phenomenon: the impact of parasitic laying worker clones in apiaries and natural populations. Apidologie 33, 233–244
Moritz, R.F.A., Fuchs, S. (1998) Organization of honeybeee colonies: characteristics and conse- quences of a superorganism concept. Apidologie 29, 7–21
Moritz, R.F., Haberl, M. (1994) Lack of meiotic recombination in thelytokous parthenogenesis of laying workers of Apis mellifera capensis (the Cape honeybee). Heredity 73, 98–102
Moritz, R.F.A., Southwick, E.E. (1992) Bees as super- organisms. Springer, Berlin
Moritz, R.F.A., Kryger, P., Allsopp, M.H. (1996) Competition for royalty in bees. Nature 384, 31
Moritz, R.F.A., Kryger, P., Allsopp, M.H. (1999) Lake of worker policing in the Cape honeybee (Apis mellifera capensis). Behaviour 136, 1079–1092
Moritz, R.F.A., Pflugfelder, J., Crewe, R.M. (2003) Lethal fighting between honeybee queens and parasitic workers (Apis mellifera). Naturwissenschaften 90, 378–381
Moritz, R.F.A., Lattorff, H.M.G., Crewe, R.M. (2004) Honeybee workers (Apis mellifera capensis) compete for producing queen-like pheromone signals. Proc. R. Soc. Lond. B. Biol. Sci. 271(Supplement 3), S98– S100
Moritz, R., Pirk, C.W.W., Hepburn, H.R., Neumann, P. (2008) Short-sighted evolution of virulence in parasitic honeybee workers (Apis mellifera capensis Esch.). Naturwissenschaften 95, 507–513
Moritz, R.F.A., Lattorff, H.M.G., Crous, K.L., Hepburn,
H.R. (2011) Social parasitism of queens and workers in the Cape honeybee (Apis mellifera capensis). Behav. Ecol. Sociobiol. 65, 735–740
Nanork, P., Paar, J., Chapman, N.C., Wongsiri, S., Oldroyd, B.P. (2005) Asian honey bees parasitize the future dead. Nature 437, 829
Nanork, P., Chapman, N.C., Wongsiri, S., Lim, J., Gloag, R.S., et al. (2007) Social parasitism by workers in queenless and queenright Apis cerana colonies. Mol. Ecol. 16, 1107–1114
Neumann, P., Hepburn, H.R. (2002) Behavioural basis for social parasitism of Cape honeybees (Apis mellifera capensis). Apidologie 33, 165–192
Neumann, P., Moritz, R.F.A. (2002) The Cape honeybee phenomenon: the sympatric evolution of a social parasite in real time? Behav. Ecol. Sociobiol. 52, 271–281
Neumann, P., Radloff, S.E., Moritz, R.F.A., Hepburn, H.R., Reece, S.L. (2001) Social parasitism by honeybee workers (Apis mellifera capensis Escholtz): host finding and resistance of hybrid host colonies. Behav. Ecol. 12, 419–428
Neumann, P., Härtel, S., Kryger, P., Crewe, R.M., Moritz, R.F.A. (2011) Reproductive division of labour and thelytoky result in sympatric barriers to gene flow in honeybees (Apis mellifera L.). J. Evol. Biol. 24, 286–294
Normark, B.B. (2003) The evolution of alternative genetic systems in insects. Annu. Rev. Entomol. 48, 397–423
Oldroyd, B.P. (2002) The Cape honeybee: an example of a social cancer. Trends Ecol. Evol. 17, 249–251
Oldroyd, B.P., Beekman, M. (2008) Effects of selection for honey bee worker reproduction on foraging traits. PLoS Biol. 6, e56
Oldroyd, B.P., Fewell, J.H. (2007) Genetic diversity promotes homeostasis in insect colonies. Trends Ecol. Evol. 22, 408–413
Oldroyd, B. P., Smolenski, A. J., Cornuet, J.-M., Crozier,
R. H. (1994) Anarchy in the beehive. Nature 371,
749
Oldroyd, B.P., Allsopp, M.H., Gloag, R.S., Lim, J., Jordan, L.A., et al. (2008) Thelytokous partheno- genesis in unmated queen honeybees (Apis mellifera capensis): central fusion and high recombination rates. Genetics 180, 359–366
Oldroyd, B.P., Allsopp, M.H., Lim, J., Beekman, M. (2011) A thelytokous lineage of socially parasitic honey bees has retained heterozygosity despite at least 10 years of inbreeding. Evolution 65, 860–868
Onions, G.W. (1912) South African ‘fertile worker bees’.
Agr. J. U. S. Afr. 1, 720–728
Otto, S.P., Gerstein, A.C. (2006) Why have sex? The population genetics of sex and recombination. Biochem. Soc. Trans. 34(Part 4), 519–522
Page, R.E. (2013) The spirit of the hive. The mecha- nisms of social evolution. Harvard University Press, Cambridge, MA
Palmer, K.A., Oldroyd, B.P. (2000) Evolution of multiple mating in the genus Apis. Apidologie 31, 235–248
Pearcy, M., Aron, S., Doums, C., Keller, L. (2004) Conditional use of sex and parthenogenesis for worker and queen production in ants. Science 306, 1780–1783
Pearcy, M., Hardy, O., Aron, S. (2006) Thelytokous parthenogenesis and its consequences on inbreeding in an ant. Heredity 96, 377–382
Phiancharoen, M., Pirk, C.W.W., Radloff, S., Hepburn,
R. (2010) Clinal nature of the frequencies of ovarioles and spermathecae in Cape worker honey- bees, Apis mellifera capensis. Apidologie 41, 129– 134
Pirk, C.W.W., Neumann, P., Hepburn, H.R. (2002) Egg laying and egg removal by workers are positively correlated in queenright Cape honeybee colonies (Apis mellifera capensis). Apidologie 33, 203–211
Rabeling, C., Kronauer, D.J.C. (2013) Thelytokous parthenogenesis in eusocial hymenoptera. Annu. Rev. Entomol. 58, 273–292
Ratnieks, F.L.W. (1988) Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am. Nat. 132, 217–236
Ratnieks, F.L.W., Visscher, P.K. (1989) Worker policing in honeybees. Nature 342, 796–797
Ravary, F., Jaisson, P. (2004) Absence of individual sterility in thelytokous colonies of the ant Cerapachys biroi Forel (Formicidae, Cerapachyinae). Insectes Soc. 51, 67–73
Ruttner, F. (1977) The problem of the cape bee (Apis mellifera capensis Escholtz): parthenogenesis—size of population—evolution. Apidologie 8, 281–294
Seeley, T.D. (1989) The honey bee colony as a superorganism. Am. Scientist 77, 546–553
Seeley, T.D. (2010) Honeybee democracy. Princeton University Press, Princeton, NJ
Seeley, T.D., Tarpy, D.R. (2007) Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. B 274, 67–72
Siddle, H.V., Kaufman, J. (2013) A tale of two tumours: comparison of the immune escape strategies of contagious cancers. Mol. Immunol. 55, 190–193
Solignac, M., Vautrin, D., Baudry, E., Mougel, F., Loiseau, A., et al. (2004) A microsatellite-based linkage map of the honeybee, Apis mellifera L. Genetics 167, 253–262
Solignac, M., Mougel, F., Vautrin, D., Monnerot, M., Cornuet, J.-M. (2007) A third-generation microsatellite-based linkage map of the honey bee, Apis mellifera, and its comparison with the sequence-based physical map. Genome Biol. 8, R66
Sumner, S., Keller, L. (2008) Social evolution: reincar- nation, free-riding and inexplicable modes of repro- duction. Curr. Biol. 18, R206–R207
Suomalainen, E., Saura, A., Lokki, J. (1987) Cytology and evolution in parthenogenesis. CRC Press, Boca Raton, FL
Tiedemann, R., Moll, K., Paulus, K.B., Schlupp, I. (2005) New microsatellite loci confirm hybrid origin, parthenogenetic inheritance, and mitotic gene conversion in the gynogenetic Amazon molly (Poecilia formosa). Mol. Ecol. Notes 5, 586–589
Tribe, G.D. (1983) What is the Cape bee? South African Bee Journal 55, 77–87
Verma, S., Ruttner, F. (1983) Cytological analysis of thelytokous parthenogenesis in the Cape honey bee Apis mellifera capensis. Apidologie 14, 41– 57
Visscher, P.K. (1989) A quantitative study of worker reproduction in honey bee colonies. Behav. Ecol. Sociobiol. 25, 247–254
Weinberg, R. (1998) One renegade cell: the quest for the origins of cancer. Phoenix, London
Wenseleers, T., Alves, D.A., Francoy, T.M., Billen, J., Imperatiz-Fonseca, V.L. (2011) Intraspecific queen parasitism in a highly eusocial bee. Biol. Lett. 7, 173–176
Wheeler, W.M. (1911) The ant-colony as an organism.
Morphology 22, 307–325
White, M. (1984) Chromosomal mechanisms in animal reproduction. Bull. Zoo. 51, 1–23
Winston, M.L. (1991) The biology of the honey bee.
Harvard University Press, Harvard
Wirtz, P., Beetsma, J. (1972) Induction of caste differ- entiation in the honey bee (Apis mellifera) by juvenile hormone. Entomol. Exp. Appl. 15, 517–520
Woyke, J. (1963) What happens to diploid drone larvae in a honeybee colony? J. Apic. Res. 2, 73–75
Zchori-Fein, E., Gottlieb, Y., Kelly, S.E., Brown, J.K., Wilson, J.M., et al. (2001) A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc. Natl. Acad. Sci. USA 98, 12555– 12560
https://cloverhoney.web.id/
https://cloverhoney.web.id/clover-honey-madu-hdi/
https://cloverhoney.web.id/propoelix/
https://cloverhoney.web.id/royal-jelly-hdi/
https://cloverhoney.web.id/clover-honey-harga/
https://cloverhoney.web.id/propoelix-harga/
https://cloverhoney.web.id/hdi-propoelix-adalah/
https://cloverhoney.web.id/manfaat-propoelix/
https://cloverhoney.web.id/madu-hdi-harga/
https://cloverhoney.web.id/propoelix-plus/
https://cloverhoney.web.id/madu-hdi-manfaatnya/
https://cloverhoney.web.id/clover-honey-manfaatnya/
0 notes
Text
Should We Grow Genetically Modified Cannabis (GMO)?
These days, humanity has the skills to splice any genes into any DNA sequence. And this, of course, is occurring to hashish too. From separating uncommon cannabinoids to developing THC-rich bacteria, the probabilities are bizarre and wonderful. But is GMO weed safe, and ought to we be the use of it?
GMOs are in all places these days, even if you had no idea. But what does GMO stand for? GMO ability genetically modified organism. These are organisms whose genes have been artificially altered to provide them sure traits.

But is there genetically modified marijuana out there? Yes, there is.
Genetic amendment is a very controversial subject. Little-understood and distinctly complex, it provides humanity and the surroundings profound opportunities, however additionally comes with profound risks. So, is GMO properly or bad? And need to we be smoking GMO weed?
What Is GMO?
GMOs are organisms produced with the aid of inserting genetic fabric (sometimes from any other species) into their very own genetic sequences to motive them to show off some applicable trait. Whatever your take on it, it’s clearly great stuff.
The World Health Organization (WHO) defines GMOs as follows: “Genetically modified organisms (GMOs) are plants, animals or microorganisms in which genetic cloth (DNA) has been altered in a way that does no longer take place naturally by means of mating and/or herbal recombination”.
There are various distinct approaches to genetically regulate an organism.
DNA Transference Through Biological Vehicles
We have to in no way underestimate the electricity of nature. There are more than a few organisms current in the herbal world that have the strength to alter the genetic sequences of others. By manipulating these functions, we are in a position to insert DNA of our desire into residing cells.
Agrobacterium tumefaciens
This is a kind of bacterium that organically mutates the cells of plants, inflicting them to develop tumours. In the 70s, it was once located that by means of deleting the bacterial plasmid that prompted this mutation and changing it with the preferred genetic sequence, Agrobacterium may want to be used to genetically engineer plants. This is one of the oldest and most broadly used techniques of genetic change for plants.
Genetic Transformation by means of Virus
This works in a comparable way to Agrobacterium. Viruses work with the aid of infecting cells with stray genetic sequences and turning them into virus factories. By manipulating the approaches in which viruses themselves manipulate cells, we are capable to implant DNA sequences of our choosing.
Direct Transference
As nicely as the use of organisms as intermediaries, we can additionally at once genetically adjust organisms. However, this method can be a little extra scattergun (literally), and tends to be much less efficient.
Gene Guns
Gene guns, or biolistic particle transport systems, hearth tiny pellets of heavy steel covered in ideal genes at the organism of interest. The ratio of success is pretty low with this method. But if successful, the pellets penetrate the cells and combine the genetic codes on the pellets with these of the cells. It can be used to exchange DNA, RNA, and proteins.
Microinjection
This is as it sounds. Genetic fabric is injected at once into the pronucleus of a telephone the use of a micropipette. Though exceptionally easy (in phrases of genetic modification), it is now not the most dependable method.
Fast, Free Cannabis Delivery and Weed Delivery, Proudly serving Richmond, San Pablo, Hercules, Rodeo, Pinole, EI Sobrante, Kensington, and Albany
0 notes
Text
Best IVF Center In Coimbatore/Best IVF Doctor in Coimbatore-Vinsfertility
IVF TREATMENT-In Vitro Fertilisation
Sperm collection and processing are to be done with great care to avoid infection. Before this IVF Treatment is designed, the husband must have a trial preparation of sperm to see the integrity and biological nature of the sperm and their survival hours. Now after egg pick up the husband is asked to give the semen sample within 2 hours. If he thinks it might be a problem in these tense hours, he must have done it during earlier days, which could be cryopreserved. The same applies to husbands working abroad or undergoing surgery/radio, chemotherapy. The husband could be given a non-toxic condom if he is unable to produce the sample by masturbation. The specifically prepared sperms and the incubated eggs are combined in a test tube or petri dish in a prescribed number ratio and left for a few hours for them to unite. The culture dishes are kept in a modular chamber which holds the gaseous environment very strictly and these are kept in turn in a CO2 incubator. Our IVF lab utilizes a TRIGAS (O2, CO2, N2 mixture) incubator instead of CO2 alone since the presence of 5 % O2 improves the culture Conditions and integrity of embryos. After 18 hours, they are examined under a stereo zoom microscope for fertilization which is obvious if two pronuclei are seen clearly. Each pronucleus denotes the decondensation of a male and female nucleus (DNA). Once they are identified, fertilization is confirmed. This day could be accounted as Day 1. After 48 hours, properly growing embryos show 4 cells inside, and after 72 hours they show 8 cell divisions. Only then they are considered as good embryos. Once the cell division seems to be insufficient and the embryos have the odd number of cells and unequal cells they are considered to be “blocked” ones and they have meager chances to make blastocysts at the end of Day 5. Depending on the number of 8 cell stage embryos available on day 3, the type of embryo transfer and the day of embryo transfer are decided. The success rates are also determined according to the number of 8 cells staged, grade I embryos available in the culture system for that particular couple.
Below is the list of IVF Centers in Coimbatore;
1, Sudha Hospitals Coimbatore
2, Sri Ramakrishna Hospital (Multi-Speciality)
3, Genesis Advanced Fertility Center
1, Sudha Hospitals Coimbatore
Sudha IVF is India's leading chain of fertility centers, providing world-class fertility treatments. We believe in evidence-based treatment and transparency in all interactions with patients. Sudha Hospitals Coimbatore has one of the best IVF centers in Coimbatore that aims at providing rapid solutions and result-oriented services to its patients. Ensuring genetic health care of all its patients, this hospital has treated over 150000 patients and continues to spread the joy of parenthood among people. The medical experts and team of researchers are thriving to move forward and improve the existing reproductive techniques to make it much easier and simpler for the patients. To provide the right guidance and treatment, they also offer counseling sessions with trained IVF experts and counselors that believe in honest report analysis. Sudha Hospitals is a leading chain of fertility centers in India, known for its commitment to excellence and patient-doctor transparency that provides the best IVF treatments with promising results. Parenthood is an astonishing stage in one’s life, and to make those dreams come true – the team here at Sudha Hospitals ensures to put their best foot forward.
2, Sri Ramakrishna Hospital (Multi-Speciality)
Sri Ramakrishna Hospital treats thousands upon thousands of patients each year. The most advanced oncological procedures to treatments for everyday ailments, we bring relief to patients from all walks of life. We use state-of-the-art technology and cutting-edge surgical and medical techniques to deliver outstanding outcomes. The original hospital is an imposing 1000-bed edifice where top-flight consultants and expertly trained staff offer advanced treatments and procedures ranging from advanced neurosurgery to chemotherapy to stem cell transplantation to organ transplants and so on. Here, driven by our founding motive of providing accessible healthcare to society, patients receive the very best of care at virtually nominal costs. With a host of ‘firsts’ to our credit over the years, we have consistently stood at the edge of medicine in the nation. We are the rare exception to the rule – a private hospital driven by motives of empathy, service to society, and excellence. At our new 230-bed super-specialty block, world-class physicians and surgeons treat patients from across the globe in more luxuriously appointed surroundings. With a high staff-patient ratio, individual attention is at its highest. Equipped with space-age medical technology and exceeding global standards, the super-specialty block outshines many of the more celebrated ‘chain hospitals’ of the metros. We’d go as far as to say it has redefined ‘corporate’ healthcare.
3, Genesis Advanced Fertility Center
Genesis IVF is an extremely distinguished medical clinic for Infertility with all facilities under one roof. It has come a long way since its inception as a pioneering institute in Coimbatore, Tamil Nadu in 1996. It has taken giant steps to become a center of excellence in all facets of infertility and IVF treatment options, which are offered at an affordable cost for the public. The success story of Genesis IVF & MMCH in infertility treatment is largely due to the support of an extremely dedicated and experienced IVF team led by Dr.Nirmala Sadasivam, who has invaluable experience due to pieces of training she had at various world-class institutes overseas, since 1991, and has pioneered in Blastocyst Culture technique since 1998. She was recognized by the National Academy of Medical Science and offered MNAMS – membership. With their vast knowledge and experience in Blastocyst culture technique, she has utilized the data of more than 4000 infertile couples treated in her center. She could do a Ph.D. in the same specialty. This institution was endorsed in its nascent stage at the National ``Art” Registry of India and is now a prestigious senior member of ISAR, India. Another feather in their cap is the inclusion of the center as one among the esteemed early participants at ASPIRE – Asia Pacific Initiation of Reproductive Endocrinology, Hong Kong, in their academic events. This institute is accredited to ICMR through the Indian Society for Assisted Reproduction.
Best IVF Doctor In Coimbatore
1, Dr. S. Dhanabagyam (MBBS, MD - Obstetrics & Gynaecology)
2, Dr. S Pradeepa Sudhakar (MBBS, DGO, DNB - Obstetrics & Gynecology)
3, Dr. Nirmala Sadasivam (MD. DGO.)
1, Dr. S. Dhanabagyam(MBBS, MD - Obstetrics & Gynaecology)
Dr. S. Dhanabagyam says "To establish a Woman Care & Infertility center and to provide world-class treatment at an affordable cost to all levels of people was my long-cherished dream and when I look back on the achievement of Sudha Test Tube Baby Center I am very comfortable and feel my dream come true. First Tube baby in Erode district at the time quality infertility treatment was available only in Chennai like a metropolitan city, was no mean achievement and when we cross the milestone of 10000 Test Tube Babies I am extremely rejoiced. I thank god, my family, colleagues, my staff, and well-wishers who have helped us to achieve this. I commit myself for the rest of my life to help this society as much as possible to overcome the stigma of infertility.
2, Dr. S Pradeepa Sudhakar (MBBS, DGO, DNB - Obstetrics & Gynecology)
Dr. S Pradeepa Sudhakar is a Gynecologist, Obstetrician, and Infertility Specialist in Purasawalkam, Chennai, and has 19 years of experience in these fields. Dr. S Pradeepa Sudhakar practices at Sudha Hospitals - Women, Child Care & Fertility Centre in Purasawalkam, Chennai and Sudha Hospitals - Women, Child Care And Fertility Centre in Siddhapudur, Coimbatore. She completed MBBS from The Tamil Nadu Dr. M.G.R. Medical University (TNMGRMU) in 2002, DGO from The Tamil Nadu Dr. M.G.R. Medical University (TNMGRMU) in 2006, and DNB - Obstetrics & Gynecology from National Board of Examination, India in 2009.Some of the services provided by the doctor are Vaginal Hysterectomy, Female Infertility Treatment, Antenatal, and Postnatal Exercise/ Physiotherapy, Gynae Laparoscopy, and Male Sexual Problems, etc.
3, Dr. Nirmala Sadasivam (MD. DGO.)
Dr. Nirmala Sadasivam The success story of Genesis IVF in infertility treatment is achieved by the IVF team led by gynecologist Dr. Nirmala Sadasivam. MD. DGO., who is trained at various world-class institutes overseas, since 1991, has pioneered in the BLASTOCYST CULTURE technique since 1998. Dr. Nirmala Sadasivam’s vast experience and consistent willingness to treat high-risk Infertility treatments, history of high success rate in a healthy pregnancy, and healthy relationship with patients have made Genesis IVF the highest choice of preference for Infertility treatments in South India.Infertility though, not life-threatening, causes intense mental agony and trauma that can only be best described by infertile couples themselves. At some point in their lives at least one in six couples will experience some degree of infertility. Established in 1996, Genesis IVF is one of the world’s leading, most experienced, and most successful fertility care institutes.
If you want to have any information related to IVF Centres and IVF Specialists, Raipur, contact us;
website
Facebook
Instagram
Linkedin
Youtube
0 notes
Note
I need non-biased scientific articles on life beginning at conception. Do you happen to have any? As many as you can please!
Of course!
My favorite is When Does Human Life Begin: A Scientific Perspective by Dr. Maureen L. Condic. (Link downloads a PDF)
If you want just some good quotes from biology and embryology textbooks, here you go:
"A zygote is the beginning of a new human being. Human development begins at fertilization."Dr. Keith L. Moore and Dr. T. V. N. Persaud,The Developing Human: Clinically Oriented Embryology
"Human development begins after the union of male and female gametes or germ cells during a process known as fertilization (conception)."Fertilization is a sequence of events that begins with the contact of a sperm (spermatozoon) with a secondary oocyte (ovum) and ends with the fusion of their pronuclei (the haploid nuclei of the sperm and ovum) and the mingling of their chromosomes to form a new cell. This fertilized ovum, known as a zygote, is a large diploid cell that is the beginning, or primordium, of a human being."[Moore, Keith L. Essentials of Human Embryology. Toronto: B.C. Decker Inc, 1988, p.2]
"The development of a human being begins with fertilization, a process by which two highly specialized cells, the spermatozoon from the male and the oocyte from the female, unite to give rise to a new organism, the zygote."[Langman, Jan. Medical Embryology. 3rd edition. Baltimore: Williams and Wilkins, 1975, p. 3]
"The development of a human begins with fertilization, a process by which the spermatozoon from the male and the oocyte from the female unite to give rise to a new organism, the zygote."[Sadler, T.W. Langman's Medical Embryology. 7th edition. Baltimore: Williams & Wilkins 1995, p. 3]
"At the moment the sperm cell of the human male meets the ovum of the female and the union results in a fertilized ovum (zygote), a new life has begun.... The term embryo covers the several stages of early development from conception to the ninth or tenth week of life."[Considine, Douglas (ed.). Van Nostrand's Scientific Encyclopedia. 5th edition. New York: Van Nostrand Reinhold Company, 1976, p. 943]
"Zygote. This cell, formed by the union of an ovum and a sperm (Gr. zyg tos, yoked together), represents the beginning of a human being. The common expression 'fertilized ovum' refers to the zygote."[Moore, Keith L. and Persaud, T.V.N. Before We Are Born: Essentials of Embryology and Birth Defects. 4th edition. Philadelphia: W.B. Saunders Company, 1993, p. 1]
"The chromosomes of the oocyte and sperm are...respectively enclosed within female and male pronuclei. These pronuclei fuse with each other to produce the single, diploid, 2N nucleus of the fertilized zygote. This moment of zygote formation may be taken as the beginning or zero time point of embryonic development."[Larsen, William J. Human Embryology. 2nd edition. New York: Churchill Livingstone, 1997, p. 17]
"Although life is a continuous process, fertilization is a critical landmark because, under ordinary circumstances, a new, genetically distinct human organism is thereby formed.... The combination of 23 chromosomes present in each pronucleus results in 46 chromosomes in the zygote. Thus the diploid number is restored and the embryonic genome is formed. The embryo now exists as a genetic unity."[O'Rahilly, Ronan and M�ller, Fabiola. Human Embryology & Teratology. 2nd edition. New York: Wiley-Liss, 1996, pp. 8, 29. This textbook lists "pre-embryo" among "discarded and replaced terms" in modern embryology, describing it as "ill-defined and inaccurate" (p. 12}]
"Almost all higher animals start their lives from a single cell, the fertilized ovum (zygote)... The time of fertilization represents the starting point in the life history, or ontogeny, of the individual."[Carlson, Bruce M. Patten's Foundations of Embryology. 6th edition. New York: McGraw-Hill, 1996, p. 3]
"[A]nimal biologists use the term embryo to describe the single cell stage, the two-cell stage, and all subsequent stages up until a time when recognizable humanlike limbs and facial features begin to appear between six to eight weeks after fertilization...."[A] number of specialists working in the field of human reproduction have suggested that we stop using the word embryo to describe the developing entity that exists for the first two weeks after fertilization. In its place, they proposed the term pre-embryo...."I'll let you in on a secret. The term pre-embryo has been embraced wholeheartedly by IVF practitioners for reasons that are political, not scientific. The new term is used to provide the illusion that there is something profoundly different between what we nonmedical biologists still call a six-day-old embryo and what we and everyone else call a sixteen-day-old embryo."The term pre-embryo is useful in the political arena -- where decisions are made about whether to allow early embryo (now called pre-embryo) experimentation -- as well as in the confines of a doctor's office, where it can be used to allay moral concerns that might be expressed by IVF patients. 'Don't worry,' a doctor might say, 'it's only pre-embryos that we're manipulating or freezing. They won't turn into real human embryos until after we've put them back into your body.'"[Silver, Lee M. Remaking Eden: Cloning and Beyond in a Brave New World. New York: Avon Books, 1997, p. 39]
208 notes
·
View notes
Text
Murine Model Organisms in Immunological Studies
Mice are a popular animal model for studying various physiological functions in the body. The mouse is a useful model because of its natural ability to succumb to diseases seen in the human population (1). They are mammals which display short generation times with many pups per litter. Mice have been used in genetic experiments for about 100 years because of the flexibility of their genome which allows for genetic modification (2). Strains of mouse have been developed with specific phenotypic characteristics which are readily available for research and are consistently genetically identical (3).
Genetic changes that have been added to the genome produce transgenic mice. Transgenic mice are of interest in studies related to studying the expression of an isolated gene from the animal of interest. The gene of interest is injected to the pronucleus of the fertilized egg which is then implanted to the uterus of a surrogate. Once born, the animal is tested for expression of the transgene. (4)
Genetic changes that have removed from the genome produce knockout mice. A gene within the embryonic stem cell (ES cells) is targeted for removal or inactivation. The ES cells are removed from the blastocyst and cultured. In culture, the target is added, and a process called electroporation is performed to enhance the uptake of the target in to the ES cell. The target is allowed to recombine with the ES cell DNA. The cells are then injected back into the embryo and the embryo is transplanted into a surrogate. (5)
https://www.genome.gov/10005834/background-on-mouse-as-a-model-organism/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2561976/
https://www.research.uky.edu/dlar/documents/CommonMouseResearchModels.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3119260/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2759098/
2 notes
·
View notes
Video
youtube
sex (sexual intercourse) 性交 せいこう
fertilisation 受精 じゅせい
conception 受胎 じゅたい
STI 性行為感染症 せいこういかんせんしょう
contraception 避妊 ひにん
sexual response 性的応答 せいてきおうとう
excitement 興奮 こうふん
plateau 定常 ていじょう
orgasm オルガズム or 性感極期 せいかんきょくき
resolution 解決 かいけつ
refractory period 不応期 ふおうき
cervical mucus 頸管粘液 けいかんねんえき
capacitate 受精能を獲得する じゅせいのうをかくとくする
acrosome 先体 せんたい or アクロソーム
granulosa cell 顆粒膜細胞 かりゅうまくさいぼう
corona radiata 放射冠 ほうしゃかん
zona pellucida 透明帯 とうめいたい
sperm receptor 精子受容体 せいしじゅようたい or 精子接受体 せいしせつじゅたい
second polar body 第二極体 だいにきょくたい
male pronucleus 雄性前核 ゆうせいぜんかく
female pronucleus 雌性前核 しせいぜんかく
mitotic spindle 紡錘体 ぼうすいたい
sterilisation (to prevent reproduction) 不妊手術 ふにんしゅじゅつ
(sterilisation to destroy microorganisms would be 滅菌法 めっきんほう)
tubal ligation 卵管結紮 らんかんけっさつ
vasectomy 精管切除術 せいかんせつじょじゅつ
barrier method バリアー法 ばりあーほう
latex ラテックス or 天然ゴム てんねんごむ
condom コンドーム
diaphragm ペッサリー
sponge スポンジ
cervical cap 子宮頚部キャップ しきゅうけいぶキャップ
female condom 女性用コンドーム じょせいようコンドーム
birth control pill 経口避妊薬 けいこうひにんやく
depo-provera デポプロベラ
contraceptive ring 避妊リング ひにん リング
intrauterine device 子宮内避妊具 しきゅうないひにんぐ
implantation 着床 ちゃくしょう
126 notes
·
View notes
Text
Episode 44 Intro | TAPP Radio Preview
Host Kevin Patton previews the content of the upcoming full episode, which focuses on how students address faculty and other topics. There's more... some word dissections, a lot of them, and a recommendation from The A&P Professor Book Club.
If you cannot see or activate the audio player click here.
Questions & Feedback: 1-833-LION-DEN (1-833-546-6336)
Follow The A&P Professor on Twitter, Facebook, Blogger, Nuzzel, Tumblr, or Instagram!
Topics
1 minute
Anatomical right and left
Semi-identical twins
Method for sorting student papers quickly
Using stickers for student feedback
How students address professors
Word Dissections
5.5 minutes
Gamification
Zygote
Tripolar
Pronucleus
Locus
Blastocyst
Book Club
5 minutes
Stiff: The Curious Lives of Human Cadavers
by Mary Roach
amzn.to/2Ys2s51
Ten Things We Use When Embalming (blog post by a funeral director, shows the little discs with hooks that keep eyelids closed) my-ap.us/2Eak1ic
Check out The A&P Professor Book Club
If the hyperlinks here are not active, go to TAPPradio.org to find the episode page.
More details at the episode page.
Transcript available at the script page.
Listen to any episode on your Alexa device.
Need help accessing resources locked behind a paywall? Check out this advice from Episode 32 to get what you need! https://youtu.be/JU_l76JGwVw?t=440
Sponsors
Transcript and captions for this episode
are supported by the
American Association of Anatomists.
anatomy.org
The Human Anatomy & Physiology Society
also provides marketing support for this podcast.
theAPprofessor.org/haps
Distribution of this episode is supported by
NYCC's online graduate program in
Human Anatomy & Physiology Instruction (HAPI)
nycc.edu/hapi
Clicking on sponsor links
helps let them know you appreciate
their support of this podcast!
Referrals also help defray podcasting expenses.
Amazon
TextExpander
Snagit & Camtasia
The A&P Professor Logo Items
Follow The A&P Professor on Twitter, Facebook, Blogger, Nuzzel, Tumblr, or Instagram!
Check out notes and transcript for this episode!
0 notes