#surfactant
Text
Lasers and Soap Films

Soap films are a great system for visualizing fluid flows. Researchers use them to look at flags, fish schooling and drafting, and even wind turbines. In this work, researchers explore the soap film's reaction to lasers. (Image and research credit: Y. Zhao and H. Xu)
Read the full article
#elasticity#flow visualization#fluid dynamics#laser#marangoni effect#physics#science#shockwave#soap film#surface tension#surfactant
71 notes
·
View notes
Text
PIPE-FREEING AGENTS AND SURFACTANT
In the dynamic world of oil and gas exploration, the efficiency and success of drilling operations are paramount. Amid the complexities of drilling through various geological formations, engineers and chemists have harnessed the power of specialized chemicals to overcome challenges. Two key players in this realm are pipe-freeing agents and surfactants, whose unique properties and applications significantly contribute to the optimization of drilling processes.

Pipe-Freeing Agents: Unleashing the Drill Pipe
Drilling for oil and gas often encounters a common adversary – the sticking of the drill pipe in wellbores. This phenomenon, known as differential sticking, arises from the formation of a filter cake on the wellbore wall. Enter pipe-freeing agents, also referred to as lubricants. These agents are designed to tackle this challenge head-on by modifying the properties of the drilling mud or fluid.
Friction Reduction
The primary function of pipe-freeing agents is to reduce friction between the drill pipe and the wellbore. This reduction in friction is essential for maintaining the smooth movement of the pipe, preventing costly delays and potential damage.
Filter Cake Modification
Pipe-freeing agents alter the composition and characteristics of the filter cake that forms on the wellbore wall. By doing so, they mitigate the risk of differential sticking, ensuring that the drill pipe can move freely through the wellbore.
Lubrication
Acting as effective lubricants, these agents ensure the seamless and efficient movement of the drill pipe. In challenging drilling conditions, where forces can impede progress, lubrication becomes a crucial factor in maintaining operational momentum.
Surfactants: The Versatile Solution in Drilling Fluids
Surfactants, with their unique amphiphilic structure, find widespread use in drilling fluids within the oil and gas industry. Their ability to interact with both water and oil makes them versatile players in addressing various challenges associated with drilling operations.
Emulsification
Surfactants play a crucial role in breaking down or stabilizing emulsions within drilling fluids. This property improves the overall stability and performance of the fluid, contributing to efficient drilling.
Wetting
Enhanced wetting properties of drilling fluids are achieved through surfactants. This ensures better coverage on solid surfaces, promoting improved drilling efficiency.
Foaming/De-foaming
Controlling the formation and elimination of foam in drilling fluids is vital for maintaining stability. Surfactants are adept at managing foam levels, preventing issues that can hinder drilling progress.
Cleaning
Surfactants assist in the removal of contaminants from surfaces within the drilling system. This cleaning action contributes to the overall effectiveness and cleanliness of the operation.
Conclusion
In the ever-evolving landscape of oil and gas exploration, the role of pipe-freeing agents and surfactants is not only pivotal but transformative. These chemical marvels, with their ability to address challenges ranging from pipe sticking to emulsion stability, are indispensable tools in the hands of drilling professionals. As technology and chemical engineering continue to advance, the development of novel formulations and the strategic application of these agents will undoubtedly remain at the forefront of efforts to optimize drilling processes and ensure the sustainability of oilfield operations.
#PIPE-FREEING AGENTS AND SURFACTANT#PIPE-FREEING AGENTS#SURFACTANT#Universal Drilling Fluids#drillings fluids
3 notes
·
View notes
Video
Bact Channels
Bacteria grow in sprawling communities – as individual cells divide, so the overall colony grows. The plucky prokaryotes share chemicals with their neighbours, often feeding growth into stubborn biofilms that are difficult to disrupt. Here researchers find another clue to survival in the colony – canals. Pictured under a microscope, this colony of Pseudomonas aeruginosa develops channels (blue) sloshing fluids along each exploratory arm of the colony as it sprawls out. Researchers find that biosurfactant chemicals made by the bacteria help to lower surface tension in the channels, allowing them to send chemical packages called vesicles, or even to travel themselves, like barges on a canal (but 100 million times smaller). The team saw this long-range transport – a form of the Marangoni effect – even in bacteria without hair-like flagella often used to waft chemicals around. Further studies may allow researchers to develop new compounds to break disease-causing colonies apart.
Written by John Ankers
Video by Ye Li and colleagues
Department of Physics, Chinese University of Hong Kong, Hong Kong, China
Video originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in eLife, September 2022
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#bacterium#bacteria#biofilms#bacterial infection#marangoni effect#vesicles#biosurfactant#surfactant#flagella#pseudomonas
10 notes
·
View notes
Photo

Soapwort (Saponaria officinalis) is a wildflower that does “exactly what it says on the tin”. This wild plant of meadows and forests across Europe and Western Asia evolved high concentrations of the chemical saponin in its stems and roots to deter herbivores. While saponins are toxic if ingested by humans and other animals, these chemicals are very useful for cleaning because they cause grease and fats to dissolve in water. These chemicals are called surfactants, and synthetic versions are found in soap, washing up liquid, shampoo, and shower gel. In the past, country folk made their own cleaning products for wool and other delicate textiles using soapwort.
#katia plant scientist#plants#wildflowers#wildflower#soapwort#saponaria#saponaria officinalis#chemistry#biochemistry#cleaning products#natural remedies#natural products#saponin#surfactant#cottagecore#cottagecore aesthetic#cottage life#english countryside#plant biology#botany
8 notes
·
View notes
Text
How to make a homemade soap for a sustainable home
Introduction
Welcome to the world of DIY! With the current environmental crisis, it is important to start making sustainable choices in our lives, and one of the easiest things we can do is create our own homemade soap. Not only is it an eco-friendly choice, but making your own soap can also be a great way to get creative and save money. In this blog post, we will be exploring how to make your own homemade soap for a sustainable home.
What is Soap?
Soap is a surfactant, which means it is a substance that decreases the surface tension of a liquid, allowing it to mix with oil and dirt. It is made from fats and oils that have been combined with an alkaline substance, such as lye or sodium hydroxide, and then left to cure. Soap can come in many forms, such as bars, liquids, and gels.
Benefits of Making Your Own Soap
Making your own soap can have many benefits. Firstly, it is a great way to save money. Store-bought soap can be expensive, especially if you are buying organic or natural products. With a few simple ingredients, you can make your own soap for a fraction of the cost.
Making your own soap is also an eco-friendly choice. Many store-bought soaps contain harsh chemicals and synthetic fragrances, both of which can be damaging to the environment. By making your own soap, you can control the ingredients that go into it, ensuring that it is free from harsh chemicals and fragrances.
Finally, making your own soap can be a great way to express your creativity. With a few simple ingredients, you can make your own unique soap. You can also customize your soap by adding natural ingredients such as essential oils, herbs, and exfoliants.
The Basics of Making Soap
Making soap is a simple process, but it does require some preparation and safety precautions. Before you begin, make sure to take the following safety precautions:
Wear protective clothing, such as long sleeves and safety goggles.
Work in a well-ventilated area.
Avoid skin contact with lye and other caustic materials.
Keep lye and other caustic materials away from children.
Once you have taken the necessary safety precautions, you can begin the process of making your own soap. The basic process for making soap is as follows:
Gather your ingredients, which may include lye, oils, butters, fragrances, and other additives.
Weigh and measure your ingredients.
Combine the lye and oil in a container and mix until the lye is fully dissolved.
Add fragrances, colors, and other additives, if desired.
Pour the mixture into a mold.
Allow the soap to cool and harden.
Unmold the soap and cut it into bars.
Allow the soap to cure for 4-6 weeks.
Tips for Making Soap
Once you have the basics of making soap down, here are some tips for making your own homemade soap:
Use a digital scale for accurate measurements.
Use a lye calculator to ensure that you are using the correct amount of lye for your recipe.
Choose natural and organic ingredients for your soap.
Use essential oils and other natural fragrances for a pleasant scent.
Use natural colorants, such as herbs, spices, and clays, to add color to your soap.
Use a mold that is appropriate for the size of your soap batch.
Allow your soap to cure for at least 4 weeks before using it.
Conclusion
Making your own soap is a great way to save money, be eco-friendly, and get creative. With a few simple ingredients, you can make your own unique soap. By following the basic process and safety precautions, you can create your own homemade soap for a sustainable home.
So, what are you waiting for? Gather your ingredients, find a mold, and get started on making your own homemade soap!
#DIY#SustainableChoices#HomemadeSoap#Creative#SaveMoney#EcoFriendly#Surfactant#FatsAndOils#AlkalineSubstance#Lye#SodiumHydroxide#Bars#Liquids#Gels#StoreBoughtSoap#Organic#Natural#HarshChemicals#SyntheticFragrances#EssentialOils#Herbs#Exfoliants#ProtectiveClothing#SafetyGoggles#SkinContact#CausticMaterials#LyeCalculator#OrganicIngredients#NaturalFragrances#NaturalColorants
0 notes
Text

#GlycerineEthoxylates have many uses as transesterification reactants, humectant and tackifier in adhesives, emulsifier to boost penetration in building and construction, synthesis of lubricants, surfactants, & silane capping, etc.
For more details, visit us at https://www.pharcos.co.in/products/glycerine-ethoxylate
0 notes
Text
Choosing a fabric for your new sofa
Choosing a fabric for your new sofa http://wp.me/p7lU3-Qv
Design decisions influence our health –so your choice of a sofa fabric could influence you and your family in ways far beyond what you imagined. Our children start life with umbilical cords infused with chemicals that affect the essence of human life itself – the ability to learn, reason and reproduce. And fabric – which cocoons us most of the time, awake and asleep – is a contributor to…
View On WordPress
#bisphenol A#body burden#CDC#Centers for Disease Control and Prevention#Dispersant#Dye#Food Rules#formaldehyde#Michael Pollan#National Institutes of Health#Polybrominated diphenyl ethers#Surfactant#United States
0 notes
Text
Non-ionic Surfactants Market - All You Need to Know About Emerging Trends & Future Forecasts
Non-ionic Surfactants Market – All You Need to Know About Emerging Trends & Future Forecasts
The non-ionic surfactants market is projected to reach USD 18.6 billion by 2027, at a CAGR of 5.1% from USD 14.5 billion in 2022. Non-ionic surfactants do not have any charge on their hydrophilic part. These surfactants carry a hydrophilic group such as alcohol, phenol, ether, ester, or amide. These are suited for the cleaning process, as they are good detergents; and are not sensitive to water…

View On WordPress
#Non-ionic Surfactant#Non-ionic Surfactant Market#Non-ionic Surfactants#Non-ionic Surfactants Market#Surfactant#Surfactants#Surfactants Market
0 notes
Text

Sodium dodecyl sulfate (SDS) is an anionic surfactant. Even though the use of SDS is significant for commercial purposes. However, this surfactant is also used in various molecular biology applications. It is a strong protein denaturant and lipid-solubilizing agent that shows denaturing action towards protein. Do you need this product for your laboratory research? Get in touch with Bio Basic inc. to get further details regarding Sodium dodecyl sulfate 20% solution.
Visit www.biobasic.com/sds-20-w-v-solution-5067 to explore more!
0 notes
Link
#2022gofm#flow visualization#fluid dynamics#marangoni effect#ocean waves#physics#plunging breaker#science#surface tension#surfactant
27 notes
·
View notes
Text

Researchers develop method for upcycling plastic waste into soap
A team led by Virginia Tech researchers has developed a new method for upcycling plastics into high-value chemicals known as surfactants, which are used to create soap, detergent, and more. The work was published in Science.
Plastics and soaps tend to have little in common when it comes to texture, appearance, and, most importantly, how they are used. But there is a surprising connection between the two on a molecular level: The chemical structure of polyethylene—one of the most commonly used plastics in the world today—is strikingly similar to that of a fatty acid, which is used as a chemical precursor to soap. Both materials are made of long carbon chains, but fatty acids have an extra group of atoms at the end of the chain.
Guoliang "Greg" Liu, associate professor of chemistry in the Virginia Tech College of Science, had long felt this similarity implied that it should be possible to convert polyethylene into fatty acids—and with a few additional steps to the process—to produce soap. The challenge was how to break a long polyethylene chain into many short—but not too short—chains and how to do it efficiently. Liu believed there was the potential for a new upcycling method that could take low-value plastic waste and turn it into a high-value, useful commodity.
Read more.
110 notes
·
View notes
Photo
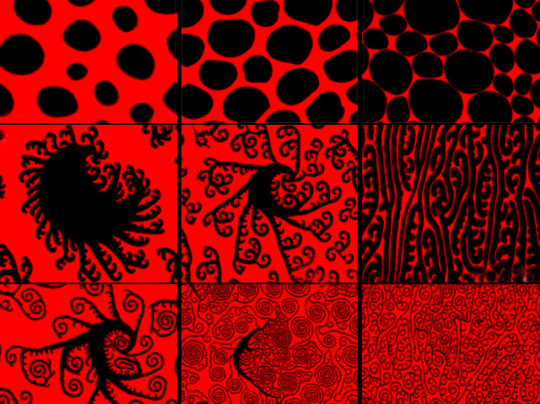
Surf’s Up
Coating our airways in a sea of shape-shifting molecules, our surfactant mixtures of proteins and fats (lipids) changes how water behaves. Pushing back against water’s natural surface tension, surfactants balance the pressure along the wet surfaces of our lungs, so they can expand and contract more easily. Infections like COVID-19 interfere with these natural mixtures, so here researchers explore how they work. Applying pressure to the blob-like lipids (left to right, top row) squeezes them together, while adding increasing amounts of cholesterol to the mix (bottom two rows) causes a spectacular change. Commonly produced by our cells, cholesterol encourages the lipid blobs to extend curly wave shapes – similar to the crystal-like growth found in snowflakes – that may help surfactant mixtures spread out as we breath. Investigating these patterns, and how they’re disrupted in disease, may be the key to new treatments tackling disorders like atelectasis.
Written by John Ankers
Image adapted from work by Cain Valtierrez-Gaytan and colleagues
Department of Chemical Engineering and Materials Science, University of Minnesota, Minneapolis, MN, USA
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Sciences Advances, April 2022
You can also follow BPoD on Instagram, Twitter and Facebook
18 notes
·
View notes
Text
Of all the damn hobby rabbitholes to fall down, it’s not fountain pen collection that gotten to me. It’s fountain pen ink formulation. Which is not a hobby I need to be getting into right now. But the thought of making my friends custom ink is soooo tempting.
#but the pretty COLORS#and i have procion dye powders in my fucking bathroom#and some automotive-grade colorshifting pigment powders???#so like. i have many of the necessary materials at my fingertips#i just need some preservatives for shelf-life purposes and some surfactants or thickeners for adjusting the flow#which. dish soap will tide me over for a surfactant and i can try gum arabic#and get some cosmetic-grade biocides for the shelf-life problem
14 notes
·
View notes
Text
friendship ended with stomach mucus, pulmonary surfactant is my new best friend
#cw medical#cw fluids#i dont think ive ever been betrayed by my pulmonary surfactant#but my stomach mucus has stabbed me in the back at least once#nightmare degenerate flesh coffin ive been consigned to by birth
3 notes
·
View notes
Text
skincare companies love lying and telling lies
#''no soap no chemicals'' girl SLS is the second quantitative ingredient listed#you are a fucking shower gel how can you be soap free#it needs the soap thats the point#anyway i am pro chemicals and pro surfactants keep me clean like michael sheen
2 notes
·
View notes