#hydrogen its compounds
Text
Program ErogenX - IT
If You Want to get it You have to maintain this Rules.
Click here & proof That you r not a Robot.
Click here

#apa itu arogan#aerogen italia#nasocad nasal drops uses#hydrogen italy#hydrogen itc#hydrogen its compounds#hydrogen is
1 note
·
View note
Text
Casually going a little feral over that one planet the james webb telescope saw that might have life on it. Like genuine potential to see extraterrestrial life. Totally absolutely normal i assure you (I'm fucking NOT)
#just to be clear it's not confirmed to have life#it might not#its just a possibility#but its a planet in the goldilocks zone with atmospheric hydrogen and likely a significant hydrosphere#and there were chemical compounds in the atmosphere that on earth are only created by living organisms#BUT this doesn't confirm anything and scientists and researchers are going to need a long time to go through all the data#and do more observations#those gasses might be made by volcanoes
0 notes
Text

What are Comet Tails Made Of? - March 26th, 1996.
"The tail of Comet Hyakutake, visible in this colour image, is composed of dust and gas driven off the icy comet nucleus by the Sun's heat and blown away by the solar wind. Bathed in solar ultraviolet light, the gas molecules break down and are excited, producing a characteristic glow. This glow is responsible for visible light from the tail, and astronomers using spectroscopes can identify the compounds involved. The close passage of Hyakutake presented an excellent chance to use this technique to explore the composition of its tail. Typical comet gas tail constituents are simple combinations of hydrogen, carbon, nitrogen, and oxygen - for example, H20 (water), CO (carbon monoxide), and CN (cyanogen) are common. In fact, the poisonous CO and CN compounds were seen in the spectrum of Halley's Comet during its 1910 apparition. This caused some public concern at the time as the Earth was expected to pass through Halley's tail! However, stretching for millions of miles, comet tails are extremely thin and tenuous and don't pose a danger to the Earth's atmosphere."
62 notes
·
View notes
Note
please please please PLEASE share more on your Thoughts about gas giants!! i'd love to learn in a way that doesnt leave me baffled and half my brain leaking from my ears! you explained things so well in the psyche post and also i think things are generally more fun to learn from someone who is Excited To Share than from Published Research Papers where everything has been dried out For Professional Reasons- understandably so, mind, but i am not In The Field and dont know the terms lol
Okay it's taken me forever to get back to this but I AM SO GLAD YOU ASKED.
Like other planets, it all starts with a disk made of gas and dust orbiting an infant star, called a protoplanetary disk. Like these in the Orion Nebula, discovered by the Hubble!

To form terrestrial planets (rocky planets with relatively thin atmospheres like Mercury, Venus, Earth, and Mars), the gas in the protoplanetary disk coalesces to form hundreds and hundreds of rocky bodies called planetesimals, about a kilometer across. These planetesimals collide, and form dozens of protoplanets about the size of the moon. The protoplanets then collide as well, and stabilize to form the solar system as we know it today.
But, in the case of gas giants, colliding protoplanets don't form fully-finished planets. Instead, they form a core, or a seed.
We think the only thing that determines whether a planet will be terrestrial or a gas giant is simply how far away from the sun it forms - that's it. As a new sun warms its evolving solar system, it heats up the material in the protoplanetary disk. Close to the sun, the disk gets hotter, and things like water and other ices melt and evaporate into gas, making them difficult for the protoplanets to gravitationally capture. However, further away, the icy compounds stay cold enough to remain solid and coalesce along with rocky particles.
That boundary in the solar system - where ices evaporate to gas on the sunward side, and remain solid on the other - is called the "Frost Line". In our solar system, the Frost Line is right between Mars and Jupiter.
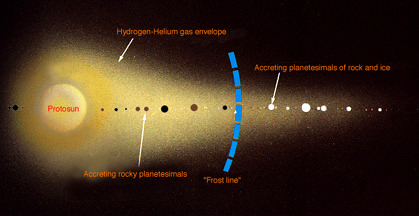
The protoplanets that form past the Frost Line turn into gas giant seeds, and are able to (kinda literally) snowball, picking up both rocky and icy material. With all that solid ice available, they grow far larger and far faster than planets in the inner solar system, and their gravity gets stronger and stronger. More gravity causes them to collect even MORE material until they're heavy enough to capture extremely lightweight elements like hydrogen and helium. Which, of course, makes them get even bigger and even heavier! Runaway growth!

But weirdly, as we study more exoplanets (planets that orbit stars other than our sun), we keep finding these huge gas giants incredibly close to their stars! Like, even closer than Mercury is to ours, which is insane. These "Hot Jupiters" break so many rules - gas giants "should" only be able to form where ice stays frozen, but here they are up close and personal with their stars, like this artist's concept!

It's possible that these planets are in the process of migrating closer to their stars, and we're managing to see them before they evaporate, but we just! Keep! Finding them!
One of my favorite parts of planetary science is how much we still have to learn. We'll think we have a pretty good idea of how things work out there, and then suddenly we'll find something that we can't explain. And there's an entire universe of weird shit - we've barely begun to scratch the surface!
#space#planets#jupiter#gas giants#astrophysics#planetary formation#Hubble#spost#asked and answered#anxiousdemifaemess#it's crazy though#in the early solar system about 98% of the material in the protoplanetary disk is hydrogen and helium#which is so lightweight it's entirely unavailable for planetary formation#it never coalesces and eventually just blows away#or gets eaten by the star#gas giants are able to utilize even a tiny fraction of that material#which is why they get so big!#The Frost Line is the reaso all our gas giants are far away and why we don't see rocky planets like mars hanging out past Jupiter and Satur#Or rather it would be#IF HOT JUPITERS WEREN'T EVERYWHERE RUINING THAT THEORY
143 notes
·
View notes
Note
here are some facts about oxygen
Animals and plants require oxygen for respiration. Plant photosynthesis drives the oxygen cycle, maintaining it around 21% in air. While the gas is essential for life, too much of it can be toxic or lethal. Symptoms of oxygen poisoning include vision loss, coughing, muscle twitching, and seizures. At normal pressure, oxygen poisoning occurs when the gas exceeds 50%.
Oxygen gas is colorless, odorless, and tasteless. It's usually purified by fractional distillation of liquefied air, but the element is found in many compounds, such as water, silica, and carbon dioxide.
Liquid and solid oxygen is pale blue. At lower temperatures and higher pressures, oxygen changes its appearance from blue monoclinic crystals to orange, red, black, and even a metallic appearance.
Oxygen is a nonmetal. It has low thermal and electrical conductivity, but high electronegativity and ionization energy. The solid form is brittle rather than malleable or ductile. The atoms readily gain electrons and form covalent chemical bonds.
Oxygen gas normally is the divalent molecule O2. Ozone, O3, is another form of pure oxygen. Atomic oxygen, which is also called "singlet oxygen" does occur in nature, although the ion readily bonds to other elements. Singlet oxygen may be found in the upper atmosphere. A single atom of oxygen usually has an oxidation number of -2.
Oxygen supports combustion. However, it is not truly flammable! It is considered an oxidizer. Bubbles of pure oxygen don't burn.
Oxygen is paramagnetic, which means it is weakly attracted to a magnet but doesn't retain permanent magnetism.
Approximately 2/3 of the mass of the human body is oxygen. This makes it the most abundant element, by mass, in the body. Much of that oxygen is part of water, H2O. Although there are more hydrogen atoms in the body than oxygen atoms, they account for significantly less mass. Oxygen is also the most abundant element in the Earth's crust (about 47% by mass) and the third most common element in the Universe. As stars burn hydrogen and helium, oxygen becomes more abundant.
Excited oxygen is responsible for the bright red, green, and yellow-green colors of the aurora. It's the molecule of primary importance, as far as generating bright and colorful auroras.
Oxygen was the atomic weight standard for the other elements until 1961 when it was replaced by carbon 12. Oxygen made a good choice for the standard before much was known about isotopes because although there are 3 natural isotopes of oxygen, most of it is oxygen-16. This is why the atomic weight of oxygen (15.9994) is so close to 16. About 99.76% of oxygen is oxygen-16.
Anon im going to killmyself please dont send me abything this long ever again i have never been more stressed scrolljng before this js too manh words im not reading all that please dont make mme read all that
41 notes
·
View notes
Text
from soil….
summary: albedo has learned many things, and yet sometimes it feels like he knows nothing at all.
word count: 3.9k
-> warnings: massive spoilers for albedo lore… bottom text
taglist: @samarill || @thenyxsky || @valeriele3 || @shizunxie || @boba-is-a-soup || @yum1x || @esthelily
< masterlist > || part 2 >>

as a synthetic human, albedo wasn’t raised as most were. he was ‘born’ fully grown, the shaky knees that let him stand those of an adult. rhinedottir hadn’t wasted any time, immediately beginning his training in various forms of alchemy from the moment he was oriented enough to try and speak.
he was taught the periodic table before he was told the names of colors, he was told how to tell which solvent was best for an experiment before he even understand the nature of his creation. he could recite the best methods for creating hydrogen gas by heart, he knew how to make carbon dioxide go supercritical and even experimented with ferrofluids on the side, but he didn’t know what it meant to be ‘burned’ until curiosity got the better of him and he put his hand over a flame.
he was told not to, like so many other things embedded in his memory, but never why. he knew fire was hot, of course, but.. even as his hand jerked away of its own accord, he found himself wondering what the odd feeling under his skin was.
rhinedottir was disappointed to learn of what he’d done, but had simply given him the instruction of ‘don’t hurt yourself, it’ll set you back.’
‘hurt’. thats what this was?
as he waited for his ammonia to drip into the iron solution, he picked through the many bookshelves in the room. many were scientific texts, with a few encyclopedias, but he wasn’t looking for those.
pulling down the lone dictionary with his now-bandaged hand, he flipped through the pages, keeping an eye on his experiment in his periphery as he did so.
hurt
(v) cause physical pain or injury to
(adj) physically injured
(n) physical injury; harm
how strange…
he shifted the book in his hands, staring at his wound through the bandages. carefully flexing his hand, he stopped right on the cusp of something sharp, the skin of his hand… was hurt.
albedo continued to read through various definitions, his experiment shifting in color to a dark brown without his notice.
why would he divert his attention from something so thrillingly new?

albedo was no longer a stranger to pain.
it took him far too long to realize he should probably be buying borosilicate glass equipment to handle the sort of experiments he was carrying out, only ever noticing when his third watchglass cracked under the heat of manganese heptoxide. his hands were permanently covered in little nicks, each carefully wrapped in bandages as to not get anything into them, some deep enough to scar but most barely enough to annoy.
slowly, he began to learn. he learned the safest ways to clean up shattered glass, he learned how to wrap his dominant hand and had become somewhat ambidextrous as a result. he learned when he needed to stop and take a break before he got a headache, he learned to tell when his hand was cramping from notes and took the time to practice with his other. pain was no longer unfamiliar, but it was still just as strange.
he was learning.
though he didn’t fully understand why this wasn’t taught to him, why he wasn’t told how to make a salve for burns or given a set of gloves to prevent it happening in the first place… he sort of could see why he wasn’t. pain was the result of failure, of a broken piece of equipment or a too-hot burner. it made sense.
did it?
he carefully poured water into a beaker, not paying attention to the conversation behind him. one of rhinedottir’s friends was over, as was becoming increasingly common, and he’d stopped listening once it turned to her daughter. a few compounds caught his attention, but he couldn’t afford to be distracted. the ratio of acetone and water had to be just right, and he was nearing the balance point, the solution fizzing less and less with every addition.
“she’s quite the- klee, don’t-”
without warning, something heavy crashed into albedo’s back. the bottle in his hand tipped and jerked, splashing into and over the rim of the beaker. the heater beneath it hissed as the ice cold water dripped down the side, and though he stood quickly, reaching to unplug it, it was too late. sparks flew as the wiring shorted, the red glow of the plate beginning to fade.
something hot and sharp rose in his chest, buzzing in his hands, the air turning thin. his jaw tightened with the feeling, the cord in his hand biting into his palm.
he’d knocked over his stool in his haste, and beside it was a small child, wide red eyes staring up at him. with bright blonde hair and long, pointed ears, it was clear she was the woman’s daughter.
and she had ran into him.
the woman—alice, his mind supplied, though he didn’t quite hear it—crouched besides her, pulling her up and dusting off her clothes, “klee! what did i say about running in the lab? you know it’s dangerous.”
rhinedottir sighed, leaning against the wall and looking at the failed experiment. “another failure…”
the sharp spikes of feeling turned on him in an instant, and the cord fell from his hand in surprise. he didn’t mean to mess it up! it wasn’t his fault klee was running around! why was he to blame?
“gold, it’s not his fault. i should have watched klee closer.”
“nonsense. he shouldn’t have even been using a bottle. pipettes are much more precise, and if he wished to have any sort of credibility to his findings, he should have used those to better track how much he was putting in. ‘add water until it stops foaming’ isn’t much of an instruction, you know.”
alice stood, some sort of response already forming in the draw of her brows, but albedo turned towards his mess. his hands shook as he moved the too-full beaker to a bin, the heating plate heavier than usual. he ignored the increasingly heated conversation behind him, letting his hands go through the familiar motions of disposal. his chest felt heavy, an odd pulse between his ribs reminding him of the reason he was wiping water off his desk.
he didn’t hold it against the girl, of course. she was too young to even be thought of chastised, and… rhinedottir was right. he probably should have used a pipette to add the water, or at least something less volatile than an open bottle. after this long, he should have known.
his vision blurred, the wad of towels in his hand washing into one mass. he threw the towels into the trash, his free hand coming up to wipe at his eyes. had vapor gotten into them? that wouldn’t be good if that were the case, but though they stung it wasn’t as sharp as it would be from chemicals.
albedo wiped up the last of the water, absentmindedly wondering why his chest ‘hurt’ if he hadn’t been injured.

alice visited often, usually bringing her daughter along as well. he wasn’t sure why, as she was surely too young to learn much in the way of alchemy, but she evidently had learned not to run in the lab, thankfully. she sat on a stool at her mother’s side, carefully drawing in a small notebook.
albedo stood at the sink, doing his best to focus on removing the caked sediment from his glassware. alice was talking, again, telling a story of a place he’d never been or heard of, and his thoughts admittedly wandered when he wasn’t careful. he’d wonder about the knights she was talking about, the cavalry led by a man in frosted blue, and he glanced over his own outfit. plain white, as typical, but he wondered about the dye that would have been used. he always wore white—“easier to tell when you’ve spilled something,” rhinedottir always said—and his few attempts at making dyes always ended up splotched and uneven. how did they dye clothes? or did they dye the thread first? would that be more or less efficient? was it harder to work with dyed thread, maybe, because it could wear during the weaving process?
curiosity bubbled within him as he rinsed off a stir rod, scraping off the leftover sediment with his nail. it would take too much time and space to try what he was thinking, not to mention that he didn’t even know how to go about it, but…
he turned to put it on a towel and paused, seeing klee looking up at him from her stool. she waved, shyly, pen tucked against her palm, and he hesitated for a moment before waving back. it was small, barely a raise of his fingers as to not draw attention, but she lit up anyway. her feet kicked against the stool in excitement and she hid her smile in her sketchbook, and albedo felt his own begin to form. he felt warm, a gentle feeling starting to rise. he tried to pin it down, running over the list of emotions he’d learned, but it didn’t match. it wasn’t the sharp, white-hot spike from when he’d ruined his hot plate, nor the slow but insistent press of curiosity. he felt… soft, almost, a delicate heat pushing him to smile back, gently-
“albedo.“
the sharp call of his name scattered the feeling like fish recessing deep into a lake, repulsed by the word.
rhine had cut off alice, evidently, the latter’s hands still raised mid-gesture.
“are you finished? why are you looking at klee like that?”
though it didn’t show on his face, albedo felt as confused as alice looked. her hands had moved to her sides, eyes flicking between the two of them with an odd twist to her mouth.
albedo swallowed something cold and bitter, taking a breath. “like what?”
he tried to put as much genuineness into his words as he could, but rhinedottir just shook her head.
“you know how.”
“i-“
“get back to work, albedo.”
she looked away, cutting the conversation short despite the argument still on his tongue.
he didn’t know. she never told him. none of the books in his lab ever described what it meant to be alive, to feel, to grow. he’d read all of them, cover to cover and back again, but none of them described what he wanted to know.
albedo turned back to the sink, wondering if there was a name for the cold pit in his stomach.

the next time alice comes, albedo has the time to look and properly greet her. he doesn’t have anything important or time sensitive going on, simply waiting for a dish to crystallize, and it was clear that the short wave he gave, pencil still in hand, had made her happy.
“hey albedo! what are you working on?”
almost subconsciously, his eyes flick to rhinedottir, searching for her approval, but she’s turned away, inspecting some random report on his desk. his chest feels cold as he lifts his sketchbook in lieu of a response. he’s drawn a cecelia, a kind of flower he saw on his last expedition, only ever growing near the top of a cliff.
he wonders of rhine would be proud of its accuracy, if nothing else.
“oh, a drawing?” klee seems to stand a bit straighter when she registers that the notebook in his hand is for drawing and not for research, and alice chuckles at her enthusiasm. “could we see?”
again, albedo seeks his master’s approval. he doesn’t find it.
he takes a quick look around the lab but knows there isn’t anything dangerous. the only active and open chemicals are the one in the beaker behind him, and that’s both well away from an edge and covered with a watchglass. so he nods, spinning his pen from his hand and into a pocket as they carefully move across the lab. he notes the caution with which klee steps over a fallen pen, the hand not in her mother’s tightly gripping her bag.
he tilts the book up for her to take—his heart had picked up at some point and he can see a quiver where his thumb digs into the binding, when did that happen?—but she just peers down at it from where she is, not reaching. it only takes a moment for something bright to reach her eyes, unfamiliar yet not unwelcome.
“cecelias, right?”
hesitantly, albedo nods. “i was exploring the eastern edge of mondstat, looking for valberries, but… i found these instead.”
she hums with a nod, her expression shifting slightly. “you need to go further north if you want valberries. cecelias grow on starsnatch cliff, and you want to go to stormbearer point.” albedo made a note to ask rhine where that was. “still, this is very impressive! the detail is remarkable despite not having a reference; you must’ve been blessed by the creator themself!”
her eyes glitter in a way that tells him it’s supposed to be something said in jest… but he doesn’t get the joke. behind her, rhinedottir’s head snapped up, eyes narrow, the report long discarded, and albedo takes the risk before his master can speak.
“who?”
alice’s face falls.

albedo looks over at klee for the nth time, checking that she was still happily doodling on her own paper. rhine had been swift to pull alice into a side room after her comment, so it was just them left in his lab. her, on the stool he’d offered her after her mother was pulled away, and him, still on the same chair he’d been for the past few hours. his pen felt cold in his hand despite the fact that he should have been producing more than enough body head to keep it warm, something… uneasy bubbling in his blood.
words pushed to the forefront of his mind, the same as they did every time he checked on klee, and this time he let them go.
“do you know who was alice talking about?”
she stops, the room falling silent as her pencil stills, and he feels oddly exposed in front of her wide red eyes. she reaches up to adjust her hat, the clover on it smudging lightly with graphite. “the creator?”
albedo nods. “rhine never calls people ‘creator’s of things, even masters of k-…. masters of alchemy are simply ‘alchemists’ to her. i’ve never heard of such a title before.”
klee pouts, stuffing her pencil into the rings on her notebook and settling it in her lap. between her fingers, he swears he sees something shaped suspiciously like a cecelia.
“the creator made everything! mama says that they are older than even her, and that they gave klee this!”
the stilted grammar of her words throws albedo off, but not as badly as when she reaches for her bag—nearly falling in the process—and unhooks a large glass-looking jewel inset in silver. it glitters red, a pattern of a flame engraved within, and he finds himself leaning closer. questions spring to his mind—‘how did you get it? what does it do? does it have a name? how is it made? how were you acknowledged by somebody so important at such a young age? is there even a significance to it at all? why doesn’t rhinedottir have one? does alice?’—but she speaks before he can voice them, voice unnaturally cohesive for somebody so young.
“i got my vision after i tried to make the biggest bomb ever!” after she what- “i made a mess out of my station… but mama says it’s okay! she helped me rebuild it and everything, and even stitched back on dodoco’s ear!” she points to a small plush charm hanging off her bag, leaving him with still more questions than answers.
“didn’t your mama teach you about them? why are you asking klee?”
albedo fell short.
was this something that parents typically taught their children? he supposed rhine would technically be his ‘mother’…. but even that was more in the literal sense. she was his mother as in she created him, but she was his master in that she taught him about and guided him through alchemy.
(but was that even for his sake? or was it hers?)
before he could say anything, alice had come back, a crease between her brows and a heavy frown on her face.
“come on klee, we’re leaving.”
klee quickly hooked the ‘vision’ back onto her bag and stuffed her notebook inside, slipping off the stood with a ‘bye bye albedo!’ before he even understood what had happened. her hand folded into her mothers, having crossed the room swiftly, free hand tucked under the strap of her bag.
alice gave albedo a long look, filled with a feeling he couldn’t begin to decipher, before her jaw set and the door opened, a wash of cold air sweeping in as they left.

rhinedottir nearly slammed open the door, shutting it just as harshly behind her, but albedo didn’t flinch from where he was weighing out sodium. she’d been returning from expeditions more and more irritated lately, the domains she’s been searching somehow turning up less clues each time. he’s not privy to her work, so he simply keeps his mouth shut, never offering his advice or help even when he knows it helps to talk puzzling things out.
he tapped his stir rod on the edge of his beaker, knocking off the excess solution, and listened to her go through her routine. boots off, shoes on, coat off, lab wear on. bag down, notes up, then the bang of her door.
he stifles a smile at her predictability. most of her actions are prescribed, a routine she likely follows unintentionally, but it brings him a small bit of comfort. she did the same things when she returned today as she did every other day, no mater the size of her discovery, retiring to her room to review her findings. he learned quickly to shut down any attention-sapping experiments as quickly as possible after she returned to be able to dedicate as much as he could to listening to her ramble, leaving space on his table for her diagrams. he rarely got a word in, but that just made him all the better listener, able to concisely say everything he wanted to in the moment’s space of her breaths.
with all of this in mind, he covered his beaker. the solution would be fine overnight, so long as it was chilled, and he was quite looking forward to tonight’s talk.
albedo stood from his stool and began to clean up, listening to the clock tick down.

a few hours later, rhine returns with a heavy sigh. he hears papers flap in her hands as she shuffles through them, the sound growing louder as she approaches. she sits in the chair he’d set out for her in preparation and drops her papers on the table in a messy pile, various diagrams drawn across them.
she picks out one seemingly at random, depicting a diamond-shaped sigil inset onto a large set of doors. a complex web of patterns wraps around it, ending on eight smaller sigils. below the diagram, she wrote out a quote, presumably the one inscribed across the top of the door, “when seeking those who have lost their faith / there’s not much one can do but wait / you take the swiftest trail at once / and try until your hopes prevail.”
he doesn’t know what it means, but he keeps the words in his mind as she shoves aside the rest of the papers, setting down that one and beginning to talk about how she tried to solve it.
“there’s over 40,000 combinations—i did the math—and i wasn’t going to sit there for however long it took. the geo slime condensate only had enough elemental energy preserved in it to activate all of the sigils twice, and that didn’t account for actually killing the things.”
albedo propped his arm on the table, resting his chin in his palm and staring at the paper. he took in and registered her words, of course, hearing and understanding them, but a majority of his mind was focused on the paper. each of the winding paths started at the center sigil and twisted out, quickly becoming hard to follow- likely due to erosion, since the domain seemed embedded into a cliff face.
still, he pulled at the puzzle, picking at the edges. the inscription played on loop in his mind, producing ideas just as quickly as he shut them down. it couldn’t be that they had to leave to a secondary—or more—location, since six separate places for a domain was too complex and highly unlikely. it couldn’t be that there was some sort of prayer or hymn they needed to follow, due to the same argument as the first. there had to be a simpler solution….
“have you tried activating them in the order of the pathways?”
silence.
he looks up at her lack of response, finding her with her hands raised, clearly mid-ramble.
“i apologize for inter-“
he’s cut off with a wave of her hand as she picks up the paper, flipping it towards her. “dont, you already said it. what do you mean by ‘order’? actually, don’t answer. you can tell me tomorrow.”
just as quickly as she arrived, rhine left, picking up all her papers and leaving with a swish of her coat, her door nearly slammed shut.
albedo’s eyes flicked to the clock. she was barely there for ten minutes.
why? he’d spoken up before… granted, never interrupted, but… surely that wasn’t a large enough offense that she left?
he looked around his desk, empty of any equipment or glassware in preparation for the usual hours-long talk. it was earlier than he normally went to sleep, and though he could in theory return to work…
an unusual hesitation had seeped under his skin, pulling at his hands when he tried to stand. what had he said to make her leave? he’d just wanted to help…
after a moment, he stood, awkwardly pushing in his stool. ‘tell her in the morning’…
something odd and unsettling curled around albedo’s limbs as he went through the motions of preparing for bed. his fingers felt stiff where he ran them through his hair, the sheets on his bed cold despite the fire. an unmovable weight had sat itself on his chest, telling him that he’d done something wrong, but couldn’t tell what.
he hadn’t done anything. he’d just offered his help. she was the one that broke routine.
the weight told him that he was wrong.
he didn’t know why.
#genshin#genshin impact#genshin sagau#genshin self aware au#sagau#self aware genshin#<- the sagau tags apply i swear#albedo#genshin albedo#gi albedo#sagau albedo#genshin alice#klee#rhinedottir#have i mentioned i HATE rhinedottir yet#the abhorrent albedo piece i have been working on. finally here.#this is a lot more of ‘character study on albedo time!!!’ than a lot of my other sagau things but. i like him so.#like even in the second part you’re only present as a statue…. but eh. my fiction my story n all that#anyway MASSIVE shout out to the genshin fan wiki. they are doing gods work and i LOVE THEM#literally heart eyes for those who moderate / upkeep the wiki. could not do my job otherwise.
278 notes
·
View notes
Text
Trophe (Greek): nourishment, food
Have you ever wondered what bacteria eat?
There are four main distinctions that are made when discussing the metabolism of bacteria. One is about whether or not they use oxygen for cellular respiration; those that do are called "aerobic", while those that use other chemicals are "anaerobic". But this is a matter for a different post: this post will be about the other three distinctions.
Often grouped together, these categories describe the methods organisms use to gain energy. They are all independent, and knowing where a bacteria falls on one of the distinctions is not nearly enough to tell you where it falls on the others. Also, all of these distinctions are spectra, and many organisms use methods from both sides.
Without further ado, I present:
Phototrophs vs. Chemotrophs
This distinction is about an organism's energy source: light, or chemical reactions? Phototrophic bacteria gain energy from photons, while chemotrophic bacteria gain energy through the oxidation of chemical compounds. In both cases, energy is gained through the transfer of an electron -- but in phototrophic organisms, this is triggered by light, while in chemotrophs it is triggered by the breaking of a chemical bond.
It's important to note that if an organism can do photosynthesis, it must be a phototroph, but not necessarily the other way around: photosynthesis requires a little something extra. All plants are phototrophs, and almost all animals are chemotrophs (and I'm pretty sure the phototrophic animals are cheating by using symbiotic bacteria).
Autotrophs vs. Heterotrophs
This distinction is about whether or not an organism can synthesize its own organic compounds. Life is carbon-based, and everyone needs to get that carbon from somewhere. Autotrophic bacteria synthesize organic compounds out of simple sources of carbon, such as carbon dioxide. Heterotrophic bacteria must source these compounds from the environment, and ultimately other forms of life.
Phototrophs can be autotrophic or heterotrophic, and likewise for chemotrophs. Remember that "something extra"? Photoautotrophs are the organisms that photosynthesize, because photosynthesis is the process of storing light energy (phototroph behavior) by converting it into chemical energy with the creation of organic compounds (autotroph behavior). Photoautotrophs form the basis of many ecosystems, with heterotrophic life relying on their by-products. They put energy into the food chain.
Organotrophs vs. Lithotrophs
This distinction is about whether or not the chemical sources of energy for an organism are organic or inorganic. Because cells get energy through electron transfer, even phototrophic organisms need chemicals to steal them from. Organotrophs source electrons from organic compounds, while lithotrophs use inorganic compounds. Both organotrophs and lithotrophs can be either autotrophic or heterotrophic.
Photolithotrophs are far more common than chemolithotrophs, and in fact, only microorganisms can be chemolithotrophs (i.e. capable of extracting energy from inorganic compounds, without the use of light). Plants are photolithotrophs; they get their energy from carbon dioxide, a simple inorganic molecule.
Lithotrophs are diverse, and while each species tends to be highly specialized, there is a wide range of chemicals on which they can feed. Various lithotrophic bacteria use ions such as sulfur, hydrogen, ammonia, iron, and carbon monoxide as their source of energy. Meanwhile, the energy sources for organotrophs are more familiar, including protein, fatty acids, and carbohydrates.
Bonus fact: human beings are chemoheteroorganotrophic! We source our energy, and our carbon molecules, from complex organic compounds that we consume.
Please enjoy this graphic that I made in MS Paint.
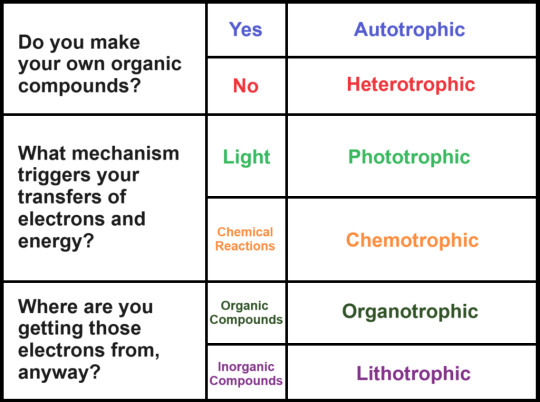
Keep an eye out for these terms when I make the bacteria propaganda posts!
28 notes
·
View notes
Text
Listen! You know I am a big Natsuhiko simp. It was normal for me to wonder about his necklace.
I think his necklace is REALLY important. What we know about Natsuhiko's necklace? It is a present from Sakura and it is germanium. He is just so casually says it in here.

I needed to check what is germanium and
1) Why did Sakura gave Natsu a germanium necklace?
Let's start with germanium itself. If I pass the more scientific stuff about germanium, this is what I get so far:
• It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin.
• Germanium is not thought to be an essential element for any living organism. Similar to silicon and aluminium, naturally-occurring germanium compounds tend to be insoluble in water and thus have little oral toxicity. However, synthetic soluble germanium salts are nephrotoxic, and synthetic chemically reactive germanium compounds with halogens and hydrogen are irritants and toxins.
•The major end uses for germanium in 2007, worldwide, were estimated to be: 35% for fiber-optics, 30% infrared optics, 15% polymerization catalysts, and 15% electronics and solar electric applications. The remaining 5% went into such uses as phosphors, metallurgy, and chemotherapy.
•Germanium supplements, made from both organic and inorganic germanium, have been marketed as an alternative medicine capable of treating leukemia and lung cancer. There is, however, no medical evidence of benefit; some evidence suggests that such supplements are actively harmful.
So. We use germanium more in technology and it is not usually harmfull for people. Wanna know what I decided to search? Germanium necklaces. Just to see if it has any meaning.
I checked the first web-site and I know, I should've search more but I was curious. I searched "germanium necklace benefits" and wanna know what I found out? Apparently, germanium jewelry;
• Increases blood circulation
• Increases metabolism of body cells
• Removes harmful toxins
•Alleviates physical stress, stiffness, cramps or discomfort
• Controls swelling
• Reduces water retention
• Relieves fatigue and tiredness
• Promotes quality sleep
• Slows down aging
• Increases and maintains body warmth
But Cesear, why are they important??
It's because, I am almost sure that Natsuhiko is not wearing his necklace in this panel.
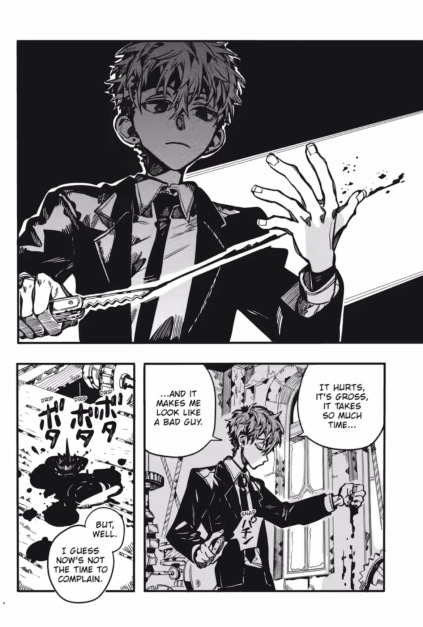
He is not wearing it. He can't. That necklace looks more like a collar than a necklace. For exemple, look here:
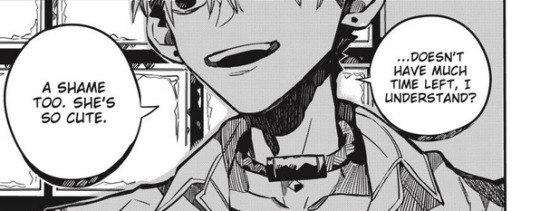
And here, now look at his germanium necklace:

That thing is not loose. I don't think he can wear that thing under a shirt that buttoned all the way up and a necktie. Look again. Do you see what I mean?

And in these panels, it is the first time Natsu talks about something that hurts him. He experienced some wild things before with Nene.

He looks scared but totally ok in here.

He looks scared, not in pain.

And he just comes back like nothing happened. These panels was one of the reasons why I thought he was immortal in the first place. He is wearing his necklace in all of them. He looks fine, he looks like his usual self. But in the raws, he looks tired. My friend pointed it out for me that in whole manga, this is the only time Natsu look genuinly tired and also, he mentions himself as a "human."
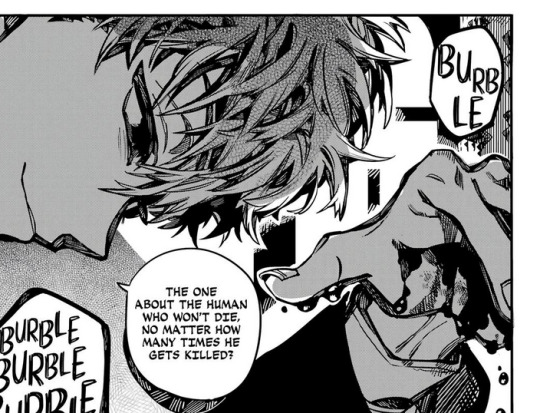
Maybe it's because he is losing blood but I don't think that much of blood and that much of a time is long enough for him to feel tired. I think he is not wearing his necklace and that necklace is healing him.
But Cesear, what does the necklace healing Natsu from?
Good question.
Before these raws, I thought that Natsu was sick and he was dying. Remember where people use germanium? Don't bother scroll back up, I'll show you again.
• Germanium supplements, made from both organic and inorganic germanium, have been marketed as an alternative medicine capable of treating leukemia and lung cancer.
But now that we have the raws, I know what the necklace heal Natsu from.
I think it is healing him from his own blood.

We now know that Natsuhiko is immortal (like I was saying for months now) because of a rumour that Sakura released but Natsu is not entirely supernatural. His blood causes the supernatural things to break down, it is toxic for them.
And if Natsu is at least somewhat immortal, that means that his blood might be toxic for him as well. He is also immortal. Which might put Natsu in a circle; his own blood keep hurting and killing him inside while his immortality forces him to live. It must be painful, he feels pain. He says that either cutting his palm or his blood hurts so he feels it. He can't be useful like that. So, how are we going to fix it?
By giving him a healing necklace which is also believed that helps with aging process.
So. Now you know why I found that necklace really important.
#toilet bound hanako kun#hyuuga natsuhiko#headcanon#i think it is really important#that necklace must mean something#it can't be just a random necklace#no fucking way
58 notes
·
View notes
Text
(the 2 most voted elements will continue onto round 2!)
More info about each element (and propaganda for the ones I like) under the cut. pleeeeeeeeease read some of them at least the one about francium
(disclaimer: these are based off short wikipedia reads and my crumbling high school chemistry knowledge. correct me if I'm wrong about anything.)
HYDROGEN: Hydrogen is the lightest element (consisting of only one proton and one electron). It is also the most abundant element in the universe, it's a gas (at room temperature) and it can explode. It's also quite representative of acids, having the (Arrhenius) definition of an acid straight up saying that it has to dissociate in water to form H+ ions. It's also quite an efficient fuel. Hydrogen is anywhere and Hydrogen is everywhere. If you like explosions, sour beverages, or acid in general, consider voting Hydrogen!
LITHIUM: Lithium, under standard conditions, is by far the least dense metal and the least dense solid element! You may primarily know him from your phone's Lithium-ion batteries. There are Lithium-based drugs used to treat mental illnesses. You can throw a block of lithium in water and it will make a really big explosion. The metal is soft and silvery. I'm running out of things to say about him. If you like batteries vote Lithium? (edit: just realised lithium is used for batteries, and batteries are connected to robotics and engineering. if you like robots and cool mechanical stuff vote lithium!)
SODIUM: You must know him from table salt. That's actually NaCl, his best known involvement. There are many more very important and very commonplace compounds that involve sodium, such as baking soda (NaHCO3) and sodium hydroxide (NaOH) (that's probably the most famous base?). It's also very important to the human body (you shouldn't eat more than 2300mg a day). If you've ever used table salt or baking soda while cooking, consider voting Sodium!
POTASSIUM: Their name was based on the word potash, which was based on an early and easy way of obtaining potassium, from putting ash in a pot, adding water, heating, and evaporating the solution. It's used in a lot of fertilisers because it's an essential plant nutrient. It's also involved in a ton of important compounds: KOH (a strong base), KNO3 (often used as salt bridges in electrochemical cells), K2CrO7 (an oxidising agent often used in organic synthesis), and K2CrO4 (I don't know what this one does). If you have ever eaten food from fertilisers consider voting Potassium!
RUBIDIUM: Rubidium compounds are sometimes used in fireworks to give them a purple color. They've also got a cool name, based on the latin rubidius, for deep red (the color of its emission spectrum). I'll be real, I don't really know much about them beyond that, but that is one cool name. Vote for Rubidium if you like cool names.
CAESIUM: Caesium is used in the definition for a second, meaning that an entire SI unit is based on it! A second can be defined as "the duration of 9,192,631,770 cycles of microwave light absorbed or emitted by the hyperfine transition of caesium-133 atoms in their ground state undisturbed by external fields". It was also discovered from mineral water. Did you know that they had to use 44000 liters of water to find her? If you've ever experienced time or had a conception of it in terms of units, consider voting Caesium!!!
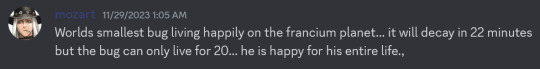
FRANCIUM!!!: Caesium... TWO! It's sad that no one will probably read this far but this is my favourite element in this poll. This element is characterised by instability. Her longest half-life is 22 minutes. Her entire existence was conjoint with Caesium before they discovered that she was her own element. She has never been seen. They literally never confirmed what color she is. She was born in a wet cardboard box all alone. Through the hands of different scientists, she was going to be named after Russia, Virginia, or Moldavia at different points in time. At one point the name catium was proposed (for "cation", since she was believed to be the most electropositive cation), but was rejected because it sounded like a cat element. Which is so fucking sad. We could've had cat element but we ended up with France element. That's right she's also named after France. Just tragic fascinating existence overall. Also isn't it just insane that her half-life is only 22 minutes? Dude, you don't get it, the most of her that's ever existed in one place is a mere 300000 atoms. She's here and she's gone. What the hell.
The charm of Francium can be summarised by the wise words of my good friend Wolfgang Amadeus Mozart:
20 notes
·
View notes
Text
Cyanide Poison

Let's start by understanding exactly how cyanide kills you. In simple terms, cyanide prevents cells from using oxygen to make energy molecules.
The cyanide ion, CN-, binds to the iron atom in cytochrome C oxidase in the mitochondria of cells. It acts as an irreversible enzyme inhibitor, preventing cytochrome C oxidase from doing its job, which is to transport electrons to oxygen in the electron transport chain of aerobic cellular respiration. Now unable to use oxygen, the mitochondria can't produce the energy carrier adenosine triphosphate (ATP). Tissues that require this form of energy, such as heart, muscle cells, and nerve cells, quickly expend all their energy and start to die. When a large enough number of critical cells die, you expire as well. Death usually results from respiratory or heart failure.
Immediate aymptoms include headaches, nausea and vomiting, dizziness, lack of coordination, and rapid heart rate. Long exposure symptoms include unconsciousness, convulsions, respiratory failure, coma and death.
A person exposed to cyanide may have cherry-red skin from high oxygen levels, or dark blue coloring, from Prussian blue (iron-binding to the cyanide ion). In addition to this, skin and body fluids may give off an almond odor.
The antidotes for cyanide include sodium nitrite, hydroxocobalamin, and sodium thiosulfate.
A high dose of inhaled cyanide is lethal too quickly for any treatment to take effect, but ingested cyanide or lower doses of inhaled cyanide may be countered by administering antidotes that detoxify cyanide or bind to it. For example, hydroxocobalamin, natural vitamin B12, reacts with cyanide to form cyanocobalamin, which leaves the body in urine.
These antidotes are administrated via injection, or IV infusion.
Cyanide is actually a lot more common than you'd think. It's in pesticides, fumigants, plastics, and electroplating, among other things. However, not all cyanide are so poisonous. Sodium cyanide (NaCN), potassium cyanide (KCN), hydrogen cyanide (HCN), and cyanogen chloride (CNCl) are lethal, but thousands of compounds called nitriles contain the cyanide group, yet aren't as toxic. They still aren't terribly good for you, so I wouldn't go around ingesting other cyanide compounds, but they're not quite as dangerous as the lethal kind.
Thank you for reading, have a lovely day :)
#cyanide#poison#cyanide poison#tw poison#poisons#chemistry#?#if it counts lmao#crime#criminal#investigation#forensics#scienceblr#science#sherlock#sherlock holmes
19 notes
·
View notes
Text
STORY AT-A-GLANCE
Antibiotics fight bacterial infections. They have no effect on infections caused by viruses, such as the common cold, seasonal influenza, SARS-CoV-2 and some ear infections
Antibiotic drugs are routinely overused, both in human medicine and agriculture, resulting in the proliferation of antibiotic-resistant infections
There are many natural plant-based remedies that will help kill bacteria without the risk of building resistance
Natural antimicrobials include medicinal honey, garlic, ginger, echinacea, goldenseal, myrrh oil, thyme essential oil, oregano oil, clove extract and cranberry juice
Remember that upper respiratory infections (URIs) are typically caused by viruses, not bacteria, so antibiotics won’t work for these infections. For URI’s, nebulized hydrogen peroxide is one of the most effective remedies. In most URI cases, improvement is seen within a few hours
As the name implies, antibiotics help fight bacterial infections. They have no effect on infections caused by viruses, such as the common cold, seasonal influenza, SARS-CoV-2 and some ear infections. Antibiotic drugs are routinely overused, both in human medicine and agriculture, resulting in the proliferation of antibiotic-resistant infections.
Taking an antibiotic unnecessarily will also kill off your beneficial gut bacteria, which could make it more difficult for you to recover from your illness. The good news is there are many natural plant-based remedies that will help kill bacteria without the risk of building resistance. Here’s a review of 10 natural antimicrobials you can reach for as a first line of defense, before resorting to a pharmaceutical antibiotic.
Medicinal Honey
As long as you use the right kind of honey, science backs its use for a variety of bacterial infections, especially when used topically. As explained in the 2011 paper, “Honey: Its Medicinal Property and Antibacterial Activity”:1
“[The] medicinal importance of honey has been documented in the world’s oldest medical literatures, and since the ancient times, it has been known to possess antimicrobial property as well as wound-healing activity.
The healing property of honey is due to the fact that it offers antibacterial activity, maintains a moist wound condition, and its high viscosity helps to provide a protective barrier to prevent infection. Its immunomodulatory property is relevant to wound repair too.
The antimicrobial activity in most honeys is due to the enzymatic production of hydrogen peroxide. However, another kind of honey, called non-peroxide honey (viz., manuka honey), displays significant antibacterial effects even when the hydrogen peroxide activity is blocked ...
The medical grade honeys have potent in vitro bactericidal activity against antibiotic-resistant bacteria causing several life-threatening infections to humans.”
In 2018, the U.K. Department of Health proposed guidelines recommending the use of honey as a first line of treatment for coughs, as part of its goal to reduce inappropriate antibiotic use. As reported by BBC News:2
“A hot drink with honey — and often with lemon and ginger as well — is a well-known home remedy for coughs and a sore throat ... [P]roposed guidelines from the National Institute for Health and Care Excellence (NICE) and Public Health England (PHE) say there is some limited evidence that it can help improve cough symptoms.”
The nectar from the manuka flower contains dihydroxyacetone, a precursor to methylglyoxal (MGO), an antimicrobial compound not found in most other honey. Australian manuka honey is perhaps the most well-known and well-studied medicinal honey, with clinical studies demonstrating its effectiveness in the treatment of:3
Bacterial infections — Manuka honey has been shown to effectively eradicate a long list of bacteria,4 including helicobacter pylori responsible for peptic ulcer diseases and gastritis, and methicillin-resistant Staphylococcus aureus (MRSA). Manuka honey also effectively removes stubborn biofilm produced by Staphylococcus aureus5
Skin diseases, ulcers, burns and necrosis
Dental caries and plaque, periodontal infections and gingivitis
Ulcerative colitis and inflammatory bowel disease (IBD)
Wounds, including post-surgical wounds
The U.S. Food and Drug Administration authorized the first manuka-based medical product in 20076 (Medihoney by Derma Sciences Inc.). Today, several different brands of Manuka-based wound and burn dressings can be found online.
Do not use conventional store-bought honey for wound care. It lacks the medicinal qualities of medicinal honeys like Manuka, and could potentially feed rather than inhibit the growth of harmful bacteria. For more information, see this September 21, 2022, article on Manuka honey. Used internally, your best bet is raw, locally sourced, unprocessed honey. There’s a lot of fake honey out there, so use caution.
Garlic
Garlic has been used to fight bacterial and parasitical infections for centuries. According to a 2014 review, garlic has been proven effective against “a plethora of gram-positive, gram-negative, and acid-fast bacteria,” including but not limited to:7
Salmonella
Escherichia coli
Pseudomonas
Proteus
Staphylococcus aureus
Klebsiella
Micrococcus
Bacillus subtIlis
Clostridium
Mycobacterium
Helicobacter
Vancomycin-resistant enterococcus
Importantly, garlic “exerts a differential inhibition between beneficial intestinal microflora and potentially harmful enterobacteria,” meaning it inhibits bad bacteria while leaving good bacteria alone.8
Garlic also has antiprotozoal and antifungal properties, and according to the 2014 review9 above, garlic can be an effective treatment for conditions such as Candida albicans, multidrug-resistant tuberculosis and giardiasis.
In the case of giardiasis — an intestinal infection marked by stomach cramps, bloating, nausea and watery diarrhea — researchers found commercially available garlic capsules eliminated the symptoms in all patients within 24 hours.
Research also supports the use of garlic and garlic derivatives for chronic external- and middle-ear infections. According to one such study,10 two components of garlic, allicin and s-allyl cysteine (SAC), were effective against the microorganisms involved in most ear infections, even at low concentrations. Garlic-infused oils are commercially available, but you can also make your own. Here’s a recipe previously published by Verywell Health:11
“You will need one garlic clove, olive oil, a pan, a strainer, a glass jar with a lid, a dropper, and a piece of cotton. You can make your own garlic oil in a few easy steps:
1.Peel and crush up the garlic.
2.Warm the oil and garlic slowly on low heat until the garlic and oil are fragrant.
3.Remove it from heat so that the mixture can cool down.
4.Strain the garlic from the oil while pouring it into a jar.
Once the oil is ready, you can use it as you would any other type of eardrop.”

Save This Article for Later - Get the PDF Now
Download PDF
Ginger
Ginger, available in extract, tincture, oil and oral capsule form, also has potent antimicrobial activity. A 2020 study12 demonstrated that ginger essential oil was effective against E. coli and S. aureus, two bacteria involved in periodontal infections.
According to the authors, the bactericidal effects of ginger essential oil appears to be due to its ability to disrupt the bacterial cell membrane. As such, it may also be useful in food preservation.
Another study13 found a 10% ginger extract effectively killed Streptococcus mutans, Candida albicans and Enterococcus faecalis, which are also implicated in the causation of oral infections. More than a dozen other bacteria are also vulnerable to its effects,14 as are a number of biofilms.15
Ginger is not suitable for children under age 2, and adults should not take more than 4 grams of ginger per day. Pregnant women are advised to cap their intake at 1 gram per day.16
Echinacea
Echinacea extract has antibacterial properties and is known to have been used by Native Americans for more than 400 years to treat wounds and infections. According to Mount Sinai Hospital,17 echinacea may be used to treat “urinary tract infections, vaginal yeast (candida) infections, ear infections (also known as otitis media), athlete's foot, sinusitis, hay fever (also called allergic rhinitis), as well as slow-healing wounds.”
It’s also a popular remedy for upper respiratory infections, such as the common cold and flu. According to a 2021 study,18 a nanosized echinacea extract was found to have up to 16-fold higher antibacterial activity against multidrug-resistant Klebsiella pneumoniae strains, compared to regular extract.
Echinacea is available in many forms, including extracts, tinctures, tablets and capsules. As a general recommendation for infection, take it three times a day for a maximum of 10 days.19
Goldenseal
Like echinacea, goldenseal is often used for the prevention and alleviation of cold symptoms, and a number of products combining the two can be found. One of the main constituents of goldenseal is berberine, known for its potent antibacterial properties.
Berberine primarily kills gram-positive bacteria, including MRSA. Berberine is not the sole component responsible for goldenseal’s antimicrobial effects, however. Extract from the aerial portions of the plant also has potent antibacterial effects that cannot be attributed to berberine alone, which is primarily found in the roots. As explained in a 2011 paper:20
“We hypothesize that [aerial goldenseal] extracts contain efflux pump inhibitors that synergistically enhance the antimicrobial activity of berberine. Bacterial efflux pumps are membrane bound proteins that pump toxins out of bacterial cells.
Overexpression of efflux pumps contributes to the development of resistance in bacteria, including S. aureus. Inhibition of efflux pumps may enhance the effectiveness of antimicrobial agents that are substrates for these pumps, and decrease the minimum inhibitory concentration for the antimicrobials.”
According to Mount Sinai,21 goldenseal is not recommended for pregnant or breastfeeding women, and those with high blood pressure, liver disease or heart disease should discuss its use with their medical provider, as it can interfere with medications prescribed for these conditions. Potential adverse effects include irritation of the skin, mouth, throat and vagina, and increased sensitivity to sunlight.
Myrrh Oil
When an antibiotic fails to kill off all the bacteria, you can end up with nongrowing bacterial persister cells. While these persisters do not undergo genetic change to make them resistant to the antibiotic, they often end up forming biofilms and are a major cause of chronic low-grade infections.22
This is where myrrh oil really shines, as research23 shows it preferentially kills off these nongrowing persister cells, and do so without the risk of promoting resistance. As noted by the authors:
“We report here that myrrh has a strong and unique antibiotic activity preferentially against nongrowing bacteria, a property not found in any commercially available antibiotic.
This unique property, along with its low toxicity and less tendency of antibiotic resistance development, suggests that myrrh can be developed to be a promising and ideal antibiotic with lower dosage requirements.”
Other research suggests it may be useful in the treatment of respiratory infections, gingivitis,24 treatment-resistant trichomoniasis vaginalis25 (a sexually transmitted disease) and Lyme disease.26
Thyme Essential Oil
Thyme essential oil has antibacterial, antibiofilm, antiviral, antifungal and antiseptic properties, and has a history of use in the treatment of upper respiratory infections. Quality matters, however. As noted in the 2020 paper, “Thymol and Thyme Essential Oil — New Insights into Selected Therapeutic Applications”:
“... only standardized preparations of thyme herb and essential oil that meet the requirements of national pharmacopeias or European Pharmacopoeia X (Ph. Eur. X) are used for the production of medicines.
According to the Ph. Eur. X definition, thyme herb is described as whole leaves and flowers separated from the dried stems of Thymus vulgaris or Thymus zygis or their mixture with 12 mL/kg of minimum essential oil (EO) and minimum thymol and carvacrol contents of 40%.
Thyme EO is defined as a product of the steam distillation of fresh flowering aerial parts of one or a mixture of both species with 37% – 55% thymol and 0.5% – 5.5% carvacrol concentrations ...
Thyme herb and its volatile oil have long been used for the treatment of upper respiratory tract infections, symptoms of bronchitis, parasitic infections, pruritus associated with dermatitis, bruises, and sprains. Nowadays, it is generally used as an expectorant in cough associated with cold and also in dentistry as a disinfectant.
It exerts an antibacterial effect on Gram-positive and Gram-negative bacteria and has antiviral (herpes simplex virus type I, human rhinoviruses and influenza viruses), antifungal, antioxidant, anti-inflammatory, and spasmolytic activity.
Although thyme volatile oil has cytotoxic properties in high concentrations and may cause intestinal cell damage when administered orally, no toxicity has been reported at commonly used doses, and it can be considered as a safe drug.
Skin administration in high concentrations may cause irritation. In rare cases, an allergic reaction can occur, manifesting as skin rash, bronchospasm, asthma attack, and anaphylaxis. Therefore, this EO is contraindicated in persons allergic to thyme or other plants from the Lamiaceae family due to a possible cross-reactivity.”
Oregano Oil
Oregano oil has shown effectiveness against bacteria such as Streptococcus mutans,27 which causes dental cavities, as well as 11 different multidrug-resistant bacteria, including Acinetobacter baumannii, Pseudomonas aeruginosa, and MRSA, and their biofilms.28
Tests have also confirmed that repeated use of oregano oil does not lead to resistance, which makes it a useful remedy in the treatment of wounds. As reported in a 2018 study in Frontiers in Microbiology:29
“While efficiently inactivating bacteria, there was no evidence of resistance development after up to 20 consecutive passages of representative bacterial strains in the presence of sublethal doses of oregano oil.
In vivo study using the third-degree burn wounds infected with PA01 or USA300 demonstrated that oregano oil, topically applied 24 h after bacterial inoculation, sufficiently reduced the bacterial load in the wounds by 3 log10 in 1 h ...
This bactericidal activity of oregano oil concurred with no significant side effect on the skin histologically or genotoxicity after three topical applications of oregano oil at 10 mg/ml for three consecutive days.
The investigation suggests potentials of oregano oil as an alternative to antibiotics for the treatment of wound-associated infections regardless of antibiotic susceptibility.”
Clove Extract and Cranberries Combat UTIs
Two natural antimicrobials shown to be useful against urinary tract infections (UTIs) are clove extract and cranberry juice. A study30 comparing the antimicrobial activity of clove extract and commercial clove essential oil (both having standardized eugenol content) found the extract was far more effective.
The ethanolic clove extract exhibited broad-spectrum inhibition against both gram-negative and gram-positive UTI-causing pathogens such as Proteus mirabilis, Staphylococcus epidermidis, S. aureus, E. coli and K. pneumoniae.
Cranberry juice is perhaps one of the most well-known remedies against UTIs. As explained in the 2018 review “Cranberry Consumption Against Urinary Tract Infections”:31
“Cranberry antibacterial effects have extensively been studied in order to understand the molecular mechanisms of action of its bioactive components and their clinical benefits against UTIs ...
Current clinical evidence clearly indicates a possible benefit overall from the use of cranberries against UTIs. Cranberry consumption may prevent bacterial adherence to uroepithelial cells, reducing UTI related symptoms.
Cranberry consumption could also decrease UTI related symptoms by suppressing inflammatory cascades as an immunologic response to bacterial invasion ... At present, cranberry supplementation can safely be suggested as complementary therapy in women with recurrent UTIs.”
A Cochrane Database of Systematic Review32 published in April 2023 confirmed that cranberry products — including juice or capsules — reduced the risk of symptomatic, confirmed UTIs in several groups.
In children, the UTI risk was reduced by 54%, in patients at increased risk to UTIs due to a medical intervention such as radiation treatment the risk was lowered by 53%, and in women with a history of recurrent UTIs the risk was reduced by 26%.
Nebulized Hydrogen Peroxide for Respiratory Infections
https://www.bitchute.com/embed/wA6P0V8hQuOK/Video Link
It’s also worth remembering what I believe is the most effective remedy for upper respiratory infections (URIs). Many make the mistake of taking antibiotics for URIs, but they won’t work, as they only kill bacteria and URIs are typically caused by viruses. The video above has my latest recommendations on how to prepare the hydrogen peroxide/saline nebulization solution and equipment to use.
Over the last three years, I’ve interviewed Dr. Thomas Levy and Dr. David Brownstein about this remarkably effective, yet simple and inexpensive treatment option. Both treated COVID patients with nebulized peroxide with great success.33,34
In most cases, including severe ones, improvement is seen within just a few hours. To inactivate viruses with hydrogen peroxide, all you need is a face mask that covers your mouth and nose and a nebulizer that emits a fine mist with properly diluted food grade hydrogen peroxide.
The microscopic mist, like smoke or vapor, can be comfortably inhaled deep into your nostrils, sinuses and lungs. I recommend using a desktop nebulizer, as they’re stronger and provide a much finer mist than handheld battery-operated versions.
Hydrogen Peroxide Rapidly Inactivates Viruses
Hydrogen peroxide (H2O2) consists of a water molecule (H2O) with an extra oxygen atom (O2), and it is the additional oxygen atom that allows it to inactivate viral pathogens. Some of your immune cells produce hydrogen peroxide to destroy pathogens. By killing the infected cell, viral reproduction is stopped. So, hydrogen peroxide therapy aids your immune cells to perform their natural function more effectively.
Many studies have investigated the use of hydrogen peroxide against different pathogens. For example, a 2020 review35 of 22 studies found that 0.5% hydrogen peroxide effectively inactivated a range of human coronaviruses, including those responsible for SARS and MERS, within one minute of exposure.
According to Brownstein, all pathogens studied to date have been found to succumb to hydrogen peroxide, albeit at varying concentrations and for different amounts of exposure.
How to Properly Dilute the Peroxide
While you can use virtually any percentage of food grade peroxide, it’s crucial to dilute it properly before use. What you want is a 0.1% dilution, so even a 3% hydrogen peroxide will need to be diluted at least 30 times.
In a pinch, you could use commercial 3% hydrogen peroxide, the stuff used for wound care, but I don’t recommend routine use of it as it contains stabilizing chemicals that can detract from the benefits. Also, you want to dilute the hydrogen peroxide with hypertonic saline, not plain water, as the lack of electrolytes in the water can damage your lungs if you nebulize that. Using saline prevents the osmotic differential that can damage lung cells.
To end up with a final peroxide/hypertonic saline solution concentration of 0.1%, you need to go through two steps:
Create the hypertonic saline solution
Dilute the peroxide
I used to recommend using normal saline, which contains 0.9% salt, but a 2021 study36 found that a 1.5% sodium chloride solution (hypertonic saline) achieved a 100% inhibition of SARS-CoV-2 replication in vitro (in cell culture). Using lower levels of saline, like 1.1%, only inhibited 88%. So, I now recommend using hypertonic saline instead, which would be slightly less than double the amount of salt used to make normal saline.
To make hypertonic (1.5%) saline, simply mix 1.5 teaspoons of high-quality unprocessed salt to one pint of purified or distilled water. Stir until the salt is thoroughly dissolved. Be sure to use proper measuring spoons and not a regular kitchen teaspoon. For even greater precision, you could use a digital scale to measure out exactly 7.1 grams of salt.
If the 1.5% hypertonic solution causes nasal burning, irritation or cough, you can lower the concentration to 0.9% salt, which is isotonic normal saline. For this you would decrease the salt to one level teaspoon to one pint of water. Once you have your saline solution and a food grade hydrogen peroxide, dilute the peroxide according to the following chart, based on the concentration you’re starting with.

!WARNING:
Food grade peroxide at concentrations of 12% and 36% should NEVER be used full-strength either topically or internally. It MUST be diluted or severe injury can occur. Your safest bet is to use 3% food grade peroxide and dilute it as indicated so you end up with a solution of 0.1%.
Once you have your peroxide-saline solution, simply pour 1 teaspoon of it into the nebulizer and inhale the entire amount. If you like, you can add one drop of 5% Lugol’s iodine solution to the nebulizer as well. Some find it boosts the effects.
I recommend using nebulized peroxide for any suspected respiratory infection, and the earlier you start, the better. If you’re already presenting with a runny nose or sore throat, use the nebulizer for 10 to 15 minutes four times a day until your symptoms are relieved.
You can also use nebulized hydrogen peroxide for prevention and maintenance, which may be advisable during flu season. There is no danger in doing it every day if you’re frequently exposed, and there may even be additional beneficial effects, such as a rapid rise in your blood oxygen level.
13 notes
·
View notes
Text
Gwynriel fic chapter titles
As I did for the Elucien fic, I'm emerging from my hiatus just to drop this thing and then going back into hibernation as soon as I hit post.
Nucleosynthesis
Prologue – Hydrogen
Chemical element with symbol H and atomic number 1. Stars such as the Sun are mainly composed of hydrogen in the plasma state.
Chapter 1 – Phosphorus
Chemical element with symbol P and atomic number 15. Its name, taken from Greek mythology, means ‘light-bearer’ (Latin Lucifer), referring to the ‘Morning Star’, the planet Venus.
Chapter 2 – Sodium
Chemical element with symbol Na and atomic number 11. It’s a highly reactive metal and an essential nutrient for many living beings.
Chapter 3 – Chlorine
Chemical element with symbol Cl and atomic number 17. It’s extremely reactive, strongly oxidizing, and corrosive.
Chapter 4 – Chromium
Chemical element with symbol Cr and atomic number 24. Its name is derived from a Greek word meaning ‘color’, since many chromium compounds are intensely colored.
Chapter 5 – Nitrogen
Chemical element with symbol N and atomic number 7. Its other suggested name, azote, comes from a Greek word meaning ‘no life’, suggesting its use as an asphyxiant gas.
Chapter 6 – Iron
Chemical element with symbol Fe and atomic number 26. Iron and iron alloys have been historically used to make weapons, and their low cost and high strength often make them the materials of choice to withstand stress or transmit forces.
Interlude – Sulfur
Chemical element with symbol S and atomic number 16. Hell is thought to ‘smell of sulfur’, likely due to its association with volcanic activity.
Chapter 7 – Fluorine
Chemical element with symbol F and atomic number 9. It’s extremely reactive, as it reacts with all other elements except for the light inert gases.
Chapter 8 – Lithium
Chemical element with symbol Li and atomic number 3. Lithium-based drugs are useful as a mood stabilizer and antidepressant in the treatment of various mental illnesses.
Chapter 9 – Argon
Chemical element with symbol Ar and atomic number 18. Its name is derived from a Greek word meaning ‘lazy’ or ‘inactive’, as a reference to the fact that this element undergoes almost no chemical reactions.
Chapter 10 – Cobalt
Chemical element with symbol Co and atomic number 27. Cobalt-based blue pigments have been used since antiquity.
Chapter 11 – Carbon
Chemical element with symbol C and atomic number 6. Its unique characteristics enable it to serve as a common element of all known life.
Chapter 12 – Oxygen
Chemical element with symbol O and atomic number 8. Many life forms require oxygen for cellular respiration, and it’s a fundamental element for combustion.
Epilogue – Helium
Chemical element with symbol He and atomic number 2, the first of the noble gases. Large amounts of new helium are created by nuclear fusion of hydrogen in stars.
Remember that Nucleosynthesis and its companion Elucien piece, Sol niger, are intricately connected.
What happens in Nucleosynthesis's prologue, interlude, and epilogue also happens in Sol niger, and these chapters share the same titles in the two stories.
#ao3#ao3 fanfic#ao3 writer#read on ao3#writeblr#my writing#writers on tumblr#fanfic#fanfiction#gwynriel#pro gwynriel#gwyn x azriel#gwynriel fic#gwynriel fanfiction#gwyneth berdara#azriel acotar#elucien#pro elucien#elucien fanfiction#elucien fic#elain archeron#lucien vanserra#acotar fanfiction#acotar#sjmaas
14 notes
·
View notes
Text
Exploring the Marvels of Biological Macromolecules: The Molecular Machinery of Life (Part 1)
In the captivating realm of biochemistry, biological macromolecules stand as the cornerstone of life itself. These intricately structured molecules, each with its unique role, orchestrate the complex symphony of biological processes. Let's dive deep into the world of macromolecules and unravel their astounding intricacies.
Carbohydrates, a group of organic compounds, are fundamental biomolecules in biochemistry. These compounds, composed of carbon (C), hydrogen (H), and oxygen (O) atoms, play multifaceted roles in various biological processes, acting as both an essential energy source and critical structural elements.
Monosaccharides: The Building Blocks
At the most basic level, carbohydrates are composed of monosaccharides, which are simple sugars. Glucose, fructose, and galactose are examples of monosaccharides. They serve as the fundamental building blocks from which more complex carbohydrates are constructed.
Polysaccharides: Storage and Structure
Carbohydrates manifest as polysaccharides, intricate macromolecules created by linking numerous monosaccharide units. Glycogen, found in animals, and starch, prevalent in plants, are storage forms of glucose. In contrast, cellulose, another glucose-based polysaccharide, forms the structural component of plant cell walls.
Energy Production: Glucose Metabolism
Carbohydrates' primary function within biological systems is to provide energy. Glucose, a hexose sugar, undergoes catabolic processes such as glycolysis and cellular respiration to generate adenosine triphosphate (ATP), the cellular energy currency. The controlled release of energy from carbohydrates fuels vital cellular functions.
Regulation of Blood Glucose: Hormonal Control
Maintaining blood glucose levels within a narrow range is crucial for homeostasis. Hormones like insulin and glucagon intricately regulate glucose levels, ensuring cells have a steady supply of this essential fuel source.
Structural Carbohydrates: Cellulose and Chitin
Carbohydrates also contribute to the structural integrity of cells and organisms. Cellulose, a linear polymer of glucose, forms the rigid cell walls of plants. Similarly, chitin, composed of N-acetylglucosamine units, provides structural support in the exoskeletons of arthropods and the cell walls of fungi.
Glycoproteins and Glycolipids: Molecular Signaling
Carbohydrates are often attached to proteins (glycoproteins) and lipids (glycolipids) on cell surfaces. These complex molecules participate in cell recognition and molecular signaling, which is crucial for various cellular processes, including immune responses and cell adhesion.

#science#biology#college#education#student#school#medicine#doctors#health#healthcare#molecules#chemistry#science nerds
24 notes
·
View notes
Text
somebody in a very long message asked me about skunks and mentioned a few other animals too so I'll do a quick post on skunks and see what I know about those other guys in later posts
also as a side note if you ever just want to chit chat about animals you can always message me or just tag me in your own posts I'm happy to have discussions as well as do these long lecture style posts :)

Skunks
cute little guys <3 I wanted a pet skunk and a pet raccoon as a kid (honestly I wanted a pet everything...) and the good news is you can technically have one! some states (17/50) will allow you to own a pet raccoon or a pet skunk but unless you are very knowledgeable in wildlife care or "exotic" pets I do not reccomend them they are not domesticated like dogs and cats are but can be tame (there's a difference)
anyways some skunk facts;
so their stinky spray is a pretty obvious skunk trait and like the messager mentioned many animals use scent as a way to communicate. pretty much all mammals have a scent gland located somewhere on their body- for many its the top of the head so they can rub pheromones off on trees or other critters to let animals know "hey this is mine" or "hey there handsome.... there's hot singles in your area"
they also mentioned possum and raccoons using scent- opossums are known for playing dead and will secrete stinky stuff to make their act more believable and raccoons? it's their urine and feces that make them stinky
some animals however use scent as a deterrent like skunks do
they are not the only animal that does this all mustelids do (that's the skink, ferret, stoat, etc. fam) and these critters are particularly stinky but don't have the spray adaptation that only skunks really have (as far as I've learned anyway)
both pet skunks and ferrets will often be surgically "de-scented" but usually still have a smell after (I mean... don't we all?)
skunks used to roll with this genus but recently made their own gang called Mephitis (literally means "stink") which has 12 species and includes skunks and "stink badgers" I've also seen some reports of 13 species but I'm not really sure off the top of my head which is true only 4 of them are "true skunks" though
skunks take their scent very seriously guys. some of them will directly aim for the eyes and others will do a little warning dance before spraying they can also adjust their sprays potency and angle and can also choose to spray from both or only one scent gland at a time

(spotted skunk- the dancers)
some can accurately spray 10 feet away but can reach up to 20 or more if they really wanted to soak you but then they have to reload for about 10 days before they can spray anything again
that odor can be smelled from 1.5 miles away! but don't worry 1/1000 humans can't smell it at all and their main predator owls also can't pick up the scent unfortunately for these birds they do still have eyes and a well aimed spray will still take them down
(also the chemical compound in their spray is flammable I have no idea who found that out and why but fun fact!)
if you ever get sprayed don't bother with tomato juice use hydrogen peroxide and baking soda to neutralize the compounds
anyways enough about stink
Skunks are omnivores and some will eat bees aiming for the actual bees over the honey like bears do (yes winny the pooh lied to you he wants that larva not necessarily the honey)
some skunks can be really social living in groups of around 10 and sometimes invite their neighbors to stay with them (there's a few cases of possums staying the night in their den) most of them ate relatively solitary but they aren't very territorial and will overlap sometimes

they are immune to snake venom! another trait that is somewhat similar to their cousins the badgers as they often eat snakes they can handle a lot of poison
alright that's what I know about skunks they're cute little guys but once again
DO. NOT. TOUCH. yes theyre stinky but they are also known to carry rabies if you see one out during the day do not approach it and call wildlife services if you are seriously worried
17 notes
·
View notes
Text
Chemistry researchers modify solar technology to produce a less harmful greenhouse gas
Researchers in the UNC-Chapel Hill Chemistry Department are using semiconductors to harvest and convert the sun's energy into high-energy compounds that have the potential to produce environmentally friendly fuels.
In the paper, "Methyl termination of p-Type silicon enables selective photoelectrochemical CO2 reduction by a molecular ruthenium catalyst," published in ACS Energy Letters, the researchers explain how they use a process called methyl termination that uses a simple organic compound of one carbon atom bonded to three hydrogen atoms to modify the surface of silicon, an essential component in solar cells, to improve its performance in converting carbon dioxide into carbon monoxide using sunlight.
The research was informed by a process called artificial photosynthesis, which mimics how plants use sunlight to convert carbon dioxide into energy-rich molecules.
Read more.
#Materials Science#Science#Chemistry#Semiconductors#Solar power#Fuel#Artificial photosynthesis#University of North Carolina
9 notes
·
View notes
Note
hey not doubting you at all, but do you have any specific proof that points to matpat being a plagiarist?
augh i had a whole thing typed up and Tumblr ate it
short version: there's been a lot of allegations for years. the hermitcraft one seems pretty cut and dry to me. he has also just stolen theories from the source material itself - when he made a theory on a series i shan't name, his whole theory was something explicitly canon in the books, and he doesn't acknowledge that once.
now for my own personal evidence. i consider this less "proof" and more "occam's razor says plagiarism, and the other explanation is that he's incredibly dumb". under cut because it involves fma and chemistry neither of which i can shut up about
so, here's the video in question:
youtube
I'd use more screenshots to prove my point here, but apparently game theory can't afford captions, so I'll just explain the old fashioned way.
Matthew is trying to prove that the Elric's formula for a human (water 35 liters, carbon 20kg, etc) is incorrect (ie. does not have the right elements in the right proportions to make a human.)
to do this, he has to convert the several compounds (water, ammonia, lime, salt, and saltpeter) into elemental masses.
first, he divides each element by its molar mass. so for water, that's 35,000g/(18g/mol)=1,942mol. this is correct. don't get used to it.
so saying you have a "mole" of something is kind of like saying you have a "dozen" of it - you know if someone has 2 dozen eggs, then they have 2x12=24 eggs, because a dozen means 12. moles are like that, except instead of 12 it's 6.023x10²³. so the moles we just calculated is the number of molecules of water that are in 35L - 1,942mol converts to ~1.17x10²⁷ molecules.
this next bit is where matthew goofs it. he says that to get from the number of water molecules to the number of oxygen atoms, you multiply the number of molecules by the percent of the weight of the water that is oxygen, so about 89%. i hope you can see why this is incorrect. (if you can't: each water molecule has one oxygen atom in it, so the number of oxygen atoms in the water is the same as the number of molecules of water, not 89% of it.) he then uses these incorrect figures to calculate the elemental masses. these are his final masses (which, for some reason, he doesn't show clearly):


but after a few jokes, he shows this table comparing the fma numbers to reality:
ignoring that the top ones are in g and the bottom in kg, you can see that he only gets the same numbers if he didn't have to do any conversion. somehow, hydrogen goes from just under 700g to 4.5kg. he doesn't explain why the numbers changed or where the new ones came from.
so, i did the maths myself (here's the full spreadsheet if you want to check my work):
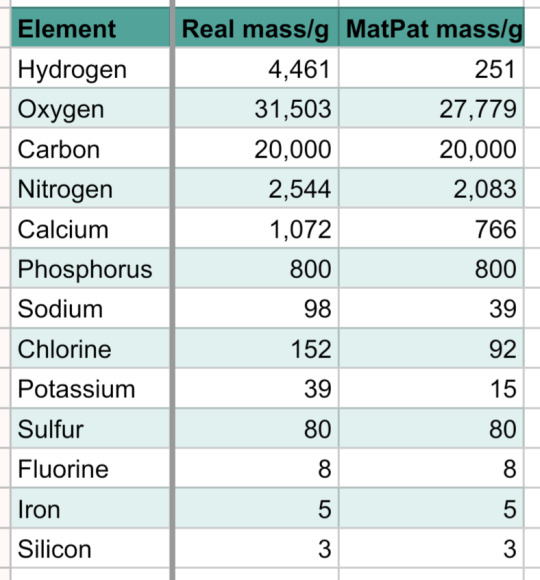
(you'll note my "matpat mass" doesn't match either set. i was doing what he said he did in the video. i have no idea what else he did to get some of his numbers. i think the hydrogen one might be because he added the correct mass from ammonia to his incorrect water mass? the oxygen i have no idea because. matthew how is there less than 3kg of oxygen in 35kg of water, even ignoring the other oxygen containing compounds)
now some of my numbers are a bit off because of rounding errors, but you'll see that my numbers agree pretty well with the wikipedia-looking chart. this is because i, unlike matpat, understand how to do chemistry calculations that they teach to babies (fourteen year olds). but the change in Matthew's numbers from two points thirty seconds apart in the video raises the question of where the hell he got them from. way i see it, there are two explanations:
1. matthew did realise he'd explained everything wrong and his maths was off, but decided to publish the video without correcting his explanation or his first set of numbers anywhere;
2. matthew stole these numbers from somewhere else, where they'd been calculated by someone who understands chemistry 101, and couldn't even understand the chemistry well enough to replicate their results.
why bother saying all of this if it proves nothing? i need you to understand that i was in chemistry for babies when this video came out, and deep in the weeds of my fma hyperfix and my chem hyperfix. this has bothered me for a full third of my life. this isn't an explanation it's an exorcism
9 notes
·
View notes