#FDA Warning Letters
Text
How the Gates Foundation Hijacks Global Health for Power and Profit
After much though and a continuing stream of new evidence coming to light, I am going to do this. This is a timeline of events leading up to and surrounding the coronavirus outbreak in late 2019. I still cannot prove that the long-lasting, breath-shortening cold many people caught in December 2019 was in anyway related to COVID-19. I also cannot find information – most likely because in 2013,…

View On WordPress
#bias#Bill and Melinda Gates Foundation#ChAdOx1#chimp adenovirus vector#communication#conflict of interest#conspiracy theorists#coronavirus#COVID-19#FDA Warning Letters#fraudulent#Gates Foundation#Ivermectin#lies#Mutant spike protein#myelitis#NHS#patents#Pirbright Institute#politics#QAnon Supporters#SARS#spinal#supplements#vaccine damage#vaccines#vitamin D#Wuhan University
26 notes
·
View notes
Text
Sometimes you just sort of remember that FDA warning letters exist and you wander over there to take a gander and then you find out that a Dollar Tree facility has been infested with rats ever since January of 2020 and only decided to permanently close the facility in May 6, 2022, and then you read a long list of inspectors just seeing all this bullshit, including finding piles of trash and old conveyor belt parts in a section of the facility apparently nicknamed the “Junk Yard” and then you just kinda stop reading for a while
99 notes
·
View notes
Text
6 Tips to avoid 483 letters
The U.S. Food and Drug Administration (FDA) is authorized to perform random inspections and audits, and these inspections can lead to FDA warning letters and FDA Form 483 observations.
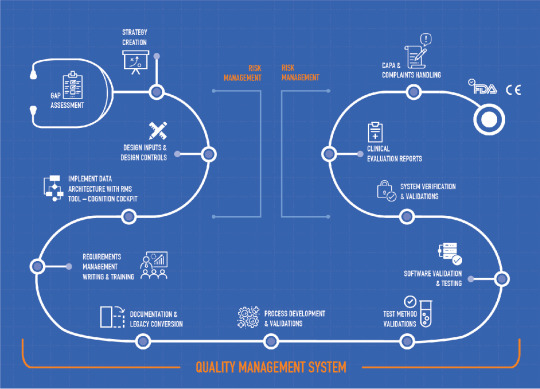
Medical device manufacturers receive observations (Form 483) and / or warning letters on completion of USFDA Audit. The document outlines any violations of Good Manufacturing Practices (GMP’s) such as the facility, equipment, processes, controls, products, employee practices or records.
Some tips to avoid a warning letter and form 483 observation –
· Be Inspection Ready
· Clearly Written Standard Operating Procedures
· Proper Document Control
· Develop a Compliance Culture
· Correct Observations Real-time
· A Hyper-adaptive Quality Management Software
IZiel has successfully assisted medical device companies to resolve these observations with efficiency and accuracy. IZiel team in collaboration with the customer utilize an extensive & comprehensive methodology to complete the remediation project. Our Onshore-Offshore Model with strong project management ensure deliveries with faster timelines, flexible resources and in cost-effective manner.
0 notes
Text
i feel like this is comfirmation bias on my end but i feel like after hearing about a bunch of deregulation of food industry shit years ago ive seen the most vile images of food online + heard about salmonella listeria e coli recalls for just about anything
#am i just more aware or has it actually gotten worse#i can find an article from science.com about how the fda issued 33% less warning letters under the trump administration (2019)#and the 2018 budget for FDA's food safety was reduced by $117 million but idk if im reading that right#its on govinfo.gov and i think is the official budget but im like#bad at reading . administrative budgets.
1 note
·
View note
Text
Data Integrity Problems as Main Topic of FDA Warning Letter
Data Integrity Problems as Main Topic of FDA Warning Letter
Data integrity continues to be one of the main topics during FDA inspections. One of the contributing causes for the warning letter to the American company Vi-Jon was the lack of controls to ensure the integrity of electronic data as well as insufficient access control to IT systems.
Based on an inspection which was carried out in October 2021 the FDA sent a warning letter to the American…

View On WordPress
0 notes
Text
I've been looking at FDA warning letters for like an hour to see what companies get cited for. they're like 90% vape stores that didn't file for permits.

hmm wonder what this one is about
53 notes
·
View notes
Text
Brenda Vise, 38 (USA 2001)

Brenda as Senior Class Secretary and Homecoming Queen in high school
It was 2001. A 38-year-old pharmaceutical representative named Brenda Colleen Vise took a pregnancy test on November 5 and it showed that she was pregnant. What the pregnancy test couldn’t tell her was that her pregnancy was ectopic.
Brenda found an advertisement for Volunteer Medical Clinic in the yellow pages. She had no idea that the deceptively named facility was actually an abortion facility that was not even licensed as a clinic. In fact, VMC had been administratively dissolved by the Tennessee Secretary of State for failure to comply with applicable law since September 17, 1999.
Staff members at VMC did another pregnancy test and then an ultrasound. Although Brenda was estimated to be 6 weeks pregnant, the ultrasound did not show a baby in the uterus. This is an obvious indicator of an ectopic pregnancy, which is a medical emergency and is a contraindication to the administration of the abortion pill.
Despite the glaring indicators of a serious condition, the VMC staff told Brenda that her ultrasound was blank because the baby was “too small to be seen.” At 6 weeks pregnant, a fetus in the uterus would have been easy to see with a competent ultrasound examination.
Brenda was given the RU-486 abortion pill and sent home with a dose of Cytotec to take by herself later. The FDA has never approved Cytotec for use in pregnant women and in fact has specifically warned against using it for an abortion. In August of the year before Brenda was killed, the manufacturer of Cytotec specifically issued a letter to healthcare providers that Cytotec was contraindicated in women who are pregnant and that Cytotec was not approved for the induction of labor or abortion, and in fact should not be used in an abortion.
After she got home, Brenda called VMC about her alarming symptoms. In Brenda’s repeated calls to VMC, she was told that her symptoms were “normal and routine.”
Brenda took the Cytotec as instructed roughly 48 hours after her initial dose of Mifeprex and continued to suffer from pain and nausea. She called VMC and told them she had a sub-normal body temperature, that she was pale and that she had significant pelvic pain. Instead of telling her to go to the hospital, VMC said that all of these were normal symptoms.
Brenda called again on September 10 in an even worse condition. She was told that her symptoms were “to be expected,” and was told to travel to VMC in Knoxville for a check-up. She was specifically told not to go to a local hospital because according to VMC, “no hospital in Chattanooga would have knowledge about the drugs that had been administered.”
Brenda’s boyfriend called an ambulance, which rushed Brenda to a Chattanooga hospital. Sure enough, her ectopic pregnancy ruptured because of VMC’s incompetence. The rupture led to a massive infection and collapse of her vital systems. On September 12 2001, Brenda was completely unresponsive and in a coma and a doctor declared her condition “terminal with no reasonable medical prospect of recovery”. She died later that day.
Even if Brenda’s baby had no chance of surviving, there was no reason that Brenda had to die too. VMC killed her with their extreme incompetence and refusal to follow medical guidelines. The state also should have better enforced the shutdown of the abortion facility that was still pretending to be a medical clinic.

33 notes
·
View notes
Text

BEES????? IN NASAL SPRAY???
#malky reads#fda warning letters#yeah hi I don’t know why but looking stuff up about brigadoon#I was just suddenly like#‘hm I haven’t seen what’s up with fda warning letters lately.’
6 notes
·
View notes
Text
If I was rich I would hire voice actors to read every single FDA warning letter from 1994-2014 so that I could listen to them like an audiobook
4 notes
·
View notes
Text
How Much Does Cannabis Oil Cost?

Whether you’re a new or experienced cannabis consumer, you’ve likely wondered, “how much does cannabis oil cost?”
This new therapeutic product has earned high praise from medical and recreational users. Each bottle of cannabis oil, also known as a tincture, comes with varying ratios of cannabinoids at relatively steep prices compared to other health- and wellness-related products.
Despite the high markup for cannabis oil, many consumers regularly use this delivery method to reap all of the mental and physical benefits of two major cannabinoids: cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC).
A number of factors affect the price of the oil. You can expect to pay anywhere from $30 to up to $200 for a single bottle. Here’s what you need to know about shopping smart for cannabis oil.
How Much Does CBD Oil Cost?
CBD oil has become widely accessible to the entire nation. It’s revered for its non-psychoactive and therapeutic effects on pain, anxiety, inflammation, seizures, and other debilitating symptoms.
The health and wellness aspect of CBD oil commands a premium price for its symptomatic relief. Apart from its medical applications, there are plenty of other factors that determine how much consumers pay for cannabis oil in general.
Katie Stem of Peak Extracts told Weedmaps, “When examining a cost analysis from a production perspective, you look at labor, materials, packaging, labels, potency/purity testing, marketing, and shipping distribution.”
For manufacturers, bulk CBD can range from $3 to $15 per gram, which works out to be less than one cent to 1.5 cents per milligram. Consumers end up paying about $50 to $60 per 1,000 mg bottle, or about 5 to 20 cents per milligram.
Why Are People Paying Premium Prices for CBD Oil?
CBD oil products, in particular, offer many potential health benefits for medical and recreational consumers. People generally buy CBD oil to help them with inflammation, pain, anxiety, stress, depression, muscle spasms, fatigue, sleep disorders, and plenty of other symptoms.
Furthermore, CBD doesn’t produce the negative side effects, especially if you take the appropriate dosage.
Despite the popularity of CBD oil products, their efficacy has not been approved by the Food and Drug Administration. Only the CBD-based drug, Epidiolex, has been approved for medical use.
In fact, many hemp companies have received warning letters from the FDA for claiming unproven health benefits on its packaging and advertising. While CBD oil can help supplement a conventional treatment plan, it’s important to consult with your physician before starting a cannabis oil regimen.
How Much Does Cannabis Oil Cost?
Cannabis oil varies in price based on cannabinoid content, as well as the region where it’s sold. Seattle-based Headset published a report detailing pricing data for a variety of marijuana products in Washington State, California, Nevada, and Colorado. The price of THC oil varied by state.
For example, Colorado had the highest price at 41 cents per milligram, which was 64 percent higher than Nevada’s 25 cents per milligram. California and Washington both had a 30 cent per milligram average price for THC oil.
Here are just a few examples of THC oil prices in the Southern California market:
- Mary’s Medicinals The Remedy THC has 1,000 mg of THC priced at $56, about 6 cents per milligram.
- Raw 1:20 THC:CBD Focus tincture has 1,000 mg priced at $87, about 9 cents per milligram.
- Select 1:1 Peppermint oil has 1,000 mg priced at $68, about 7 cents per milligram.
- Care by Design 8:1 CBD-rich sublingual drops has about 240 mg priced at $40, about 16 cents per milligram.
- Humboldt Apothecary Relax CBD 3:1 tincture has 250 mg priced at $65, about 26 cents per milligram.
- Releaf 1:1 CBD:THCa tincture has 900 mg priced at $99.62, about 11 cents per milligram.
Marijuana vs. Hemp-Derived Cannabis Oil

Cannabis oil products can be derived from either marijuana or hemp plants. Both belong to the same Cannabis sativaplant species. Marijuana plants are primarily bred for a THC-rich resin, while hemp plants produce high-CBD resin with only trace amounts of THC.
Hemp-derived oil tends to be more affordable than marijuana-derived oil. When shopping for cannabis oil, consumers may run across terms such as full-spectrum, broad-spectrum, or distillate. Each comes with varying price points depending on many factors, including its source.
Full-spectrum products contain the original chemical profile of a strain, including THC, CBD, and terpenes. Broad-spectrum contains everything in the plant but the THC, for a non-intoxicating experience. Distillates only contain one cannabinoid, either CBDA or THCA. The compounds in full-spectrum and broad-spectrum cannabis oil not only add to the aroma, but also the effects and the price.
Research into cannabinoids indicate that the interaction between different cannabinoids and terpenes produces an “entourage effect.” This synergistic effect of the plant’s compounds is thought to enhance the therapeutic benefits of a cannabis product.
For this reason, many medical consumers look for full- or broad-spectrum cannabis oil. However, someone who doesn’t want the aroma of intoxication of cannabis, may stick with a CBD isolate.
Hemp-derived CBD oil is more widely available than cannabis-derived tinctures. Ever since the 2018 Farm Bill passed, hemp-derived CBD is legal all over the country.
If you’re hoping to buy cannabis-derived tinctures, you must live in a state that allows medical cannabis (at the very least). In these states, cannabis-derived tinctures tend to be pricier because hemp isn’t as expensive to produce.
Factors Affecting Cannabis Oil Costs
A bottle of cannabis oil can vary in price based on an assortment of factors from production to marketing costs. For example, cannabis oil made from organically grown hemp from Colorado will have a higher price than oil made from a plant grown in a state with a newer market.
Besides quality, potency also affects the price of a product. Cannabis oil with 1,000 mg of cannabinoids will be more expensive than oil with fewer cannabinoids per milliliter.
The cannabis industry has unique costs and challenges that can drive up the price of cannabis oil. For example, lab testing requirements can force companies to spend hundreds of thousands of dollars testing their oil for contaminants.
Lab testing can range from $100 to $400 per sample tested. In many cases, cannabis must be tested various times throughout the supply-chain process.
Furthermore, the cannabis industry can’t write off business expenses because according to the US federal government, the marijuana plant is a Schedule I drug with no medicinal value.
Dispensaries and producers may hike up their prices to offset some of these overhead costs. Industry experts believe that full legalization will help build a stronger regulatory framework for the industry to benefit both companies and consumers.
Is Cannabis Oil Lab Tested?
Certified laboratories can provide a complete analysis of licensed cannabis product samples. Third-party labs can test for potency including its cannabinoid and terpene profile.
Labs also test for pesticides, microbial contamination, residual solvents, and other harmful chemicals that can remain after the extraction process.
Essentially, lab testing ensures the product you are buying has the potency listed on the label. More importantly, lab testing ensures the product you are consuming has no harmful contaminants that can offset its therapeutic effects.
Lab testing can significantly increase the price of cannabis oil products. However, it’s up to you to make sure your product is actually lab tested. Most companies who lab test provide a certificate of analysis (COA) on its website. Simply type in the batch number found on the packaging into their lab results page.
Buying from a licensed cannabis retailer is one of the only ways to ensure you are getting a product tested by a third-party lab. While buying hemp-derived CBD oil online without lab testing may be cheaper, we recommend you spring the extra few bucks for peace of mind and security.
How to Find Reliable and Cost-Effective Cannabis Oil

Finding the right cannabis oil at the right price point can seem like an impossible task. Luckily, there are a few ways you make sure you get the most for your dollar based on your desired results.
It can take a few hours, days, or weeks of research to find the right cannabis oil. While price matters, some affordable cannabis oils can be just as effective as the most expensive cannabis oils. Here are a few ways to save money on cannabis oil.
- Buy cannabis oil in bulk. Larger quantities mean more upfront costs, but the product often comes with considerable savings of up to 40 percent per milligram. Manufacturers pass their savings on packaging onto you. Buying in bulk can also earn you free shipping with most hemp-derived oil companies.
- Follow your favorite cannabis oil companies or retailers on their social media channels to scope out special discounts, promotions, and giveaways.
- Sign up for low-income, veteran, or other financial assistance programs if you qualify. Not every company offers this perk, but the ones that do may give you a discount of more than half off if you can send qualifying proof or apply for a spot in their program.
- Buy based on price-per-milligram. In order to calculate the price per milligram of a cannabis oil bottle, divide the total price of the product by the milligrams of cannabinoids in the product.
- When searching for bargains, always make sure you buy cannabis oil that has a certificate of analysis (COA) from an accredited third-party laboratory ensuring you have a safe and pure products.
Will CBD Prices Ever Come Down?
Industry insiders believe the price of cannabis oil will eventually go down, but not anytime soon. The industry’s strict regulations place an enormous burden on cannabis companies in terms of testing, taxes, and other rules on the plant’s production.
A variety of factors serve to limit the amount of cannabis production possible. Whether it’s commercial cannabis bans in your town or excessive licensing costs, it takes a lot of money to start up a cannabis company.
Cannabis oil may never be the most affordable natural medicine available, at least compared to pharmaceutical or herbal supplement products. However, prices are expected to go down as lawmakers become more supportive of the industry.
Once they remove the harsh limits imposed on weed companies, maybe then will the prices become accessible for those who truly need it.
As you can see, the price of cannabis oil varies widely based on the source, quality, potency, location, size, and other manufacturing and marketing costs associated with the product.
The novelty of the industry and a lack of regulation have contributed to cannabis oil’s high prices, but consumers are hopeful that one-day cannabis oil can reach an accessible price point for everyone that needs it.
Stay tuned to the Cannabis Training University blog for updates on:
- price of cannabis oil
- THC oil cost
- how much does CBD oil cost
- Colorado cannabis oil cost
- THC oil price per gram
- how to ingest cannabis oil
- cost of CBD oil products
- cheapest full-spectrum CBD oil
Learn to Grow High CBD Cannabis
There's never been a better time to learn to grow with legalization efforts ramping up worldwide! Enroll in Cannabis Training University to learn how to grow your own medicine so you can control your budget.
Become the next great cannabis grower with online cannabis training from the #1 rated marijuana school.
Read the full article
8 notes
·
View notes
Text
Published: Jun 27, 2023
Homeopathic drugs have an unusual status in the United States. On the one hand, they are incorporated into the Federal Food, Drug, and Cosmetic Act (FD&C Act) within the definition of “drug,” which specifically includes articles recognized in the official Homoeopathic Pharmacopoeia of the United States (a historical perspective can be found in this ScienceInsider article from 2015, when government scrutiny was beginning to increase). But on the other hand, there is growing consensus that the effectiveness of such products is not supported by scientific evidence and that they are, in many cases, mere placebos that do not actually treat the patient’s medical conditions; in the worst cases, they contain harmful ingredients that may cause serious injury.
This extraordinary dichotomy has led to both the Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) in recent years issuing modernized enforcement policies related to homeopathic drugs. An FTC enforcement policy statement from late 2016 requires homeopathic products to be marketed with clear disclosures stating that, among other things, there is no scientific evidence that the products work (see our prior post on the FTC policy here). Then in 2019 FDA took action to withdraw a long-standing compliance policy guidance for homeopathic drugs and to simultaneously issue a significant number of Warning Letters to companies marketing such products in violation of the FD&C Act (our prior posts on those activities are here and here).
Most recently, FDA finalized its draft guidance on homeopathic drugs – first issued in draft form in 2017 and then revised in 2019 – to lay out for industry the agency’s approach to “prioritizing regulatory actions for homeopathic products posing the greatest risk to patients.” The final guidance document issued in December 2022 can be found here. FDA also appears to be moving aggressively on the enforcement priorities as five letters relating to violative homeopathic drug products have been posted to the agency’s public Warning letter database since the beginning of calendar year 2023, as compared to four for the entire previous year. The FTC also included homeopathic drug manufacturers and distributors in the list of advertisers that received notices in April 2023 that their advertising claims need to be backed up with appropriate and reliable forms of scientific evidence (see here). Taken together, it’s clear that the homeopathy industry remains under major scrutiny by federal regulators seeking to enforce their fundamental public safety mandates, whether they fall under the FD&C Act or the prohibition on deceptive advertising contained in the Federal Trade Commission Act.
Perhaps more noteworthy and concerning for the homeopathy industry, however, is a Fall 2022 decision by the District of Columbia Court of Appeals to allow civil cases to proceed against two retail pharmacies under a plaintiff’s novel application of D.C.’s Consumer Protection Procedures Act. The plaintiff in both lawsuits is the Center for Inquiry (CFI), a nonprofit that states it is “dedicated to defending science and critical thinking in examining religion. CFI’s vision is a world in which evidence, science, and compassion – rather than superstition, pseudoscience, or prejudice – guide public policy.” As part of this mission, and among several other lawsuits it has initiated in the homeopathy space, CFI sued two retail pharmacies in the District of Columbia on the grounds that they were violating the local deceptive trade practice statute. The complaint alleged these violations arose through the pharmacies’ indirect representations that homeopathic drug products labeled as cough, cold, and flu treatments have the same characteristics and benefits as over-the-counter drug products formulated with traditional active ingredients. In particular, although the pharmacies didn’t make express promotional statements comparing the different product types, the plaintiff argued that they placed homeopathic products adjacent to their traditional counterparts on physical shelves and in online shopping results, thereby creating the misleading impression that the different products had comparable efficacy.
CFI’s complaints were dismissed at the trial court level for failure to state a claim upon which relief could be granted. The two cases were then consolidated for purposes of the plaintiff’s appeal to the D.C. Court of Appeals. On the question of whether a cognizable claim had been asserted (this post won’t discuss the separate question that the appellate court reviewed, which was whether CFI had standing to sue the defendants), a three-judge panel ruled on September 29, 2022 that “whether the complained-of practices have a tendency to mislead reasonable consumers is a jury question” – thereby reinstating the complaints and remanding the cases for factual development. In reaching its decision, the court determined that a defendant did not need to make verbal statements in order for a “representation” to exist and that actions could also fall within the scope of the deceptive trade practices statute. Therefore the various factual allegations in CFI’s complaints – for example that the pharmacies displayed homeopathic products next to “science-based” drug products and that signage in the stores indicated that the entire section contained products for “Cold, Cough & Flu Relief” – were sufficient at the pleading stage to survive a motion to dismiss. As of June 2023, the dockets for both of these CFI lawsuits are active and discovery appears to be ongoing, so they continue to bear watching for future resolution on the merits.
This recent ruling from the D.C. Court of Appeals foreshadows the possibility that retailers may opt to stop carrying homeopathic products in their stores (both physical and online) if the risk of liability to their own businesses becomes too great. Between the tightening of FDA’s and FTC’s rules for the industry and the increasingly creative use of existing consumer protection statutes by legal advocates, we could be witnessing a slow-motion demise of direct-to-consumer-based homeopathic product marketing. Only time will tell how the industry evolves in response to these numerous and formidable headwinds.
#homeopathy#Center for Inquiry#fake medicine#pseudoscience#pseudoscientific bullshit#water#literally water#woo#actual medicine#alternative medicine#Food and Drug Administration#Federal Trade Commission#homeopathy is fraud#fraud#religion is a mental illness
5 notes
·
View notes
Text
U.S. health agencies have sent a letter to Florida’s surgeon general, warning him that his claims about COVID-19 vaccine risks are harmful to the public.
The letter from the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention was sent Friday to Florida Surgeon General Joseph Ladapo. It was a response to a letter Ladapo had written the agencies last month, expressing concerns about what he described as adverse effects from mRNA COVID-19 vaccines.
Ladapo was appointed by Republican Gov. Ron DeSantis in 2021 and has attracted national scrutiny over his close alignment with the Governor in opposing COVID-19 vaccine mandates and other health policies embraced by the federal government.
Ladapo last year released guidance recommending against COVID-19 vaccinations for healthy children, contradicting federal public health leaders whose advice says all kids should get the shots.
He also has recommended against men ages 18 to 39 getting the mRNA COVID-19 vaccines, claiming that an analysis by the Florida Department of Health showed an 84% increase in cardiac-related deaths.
In their letter, the federal agencies debunked the analysis’ conclusion, saying that cardiovascular experts who studied the concern had concluded that the risk of strokes and heart attacks was lower in people who had been vaccinated, not higher.
More than 13 billion doses of COVID-19 vaccines have been given around the world with little evidence of adverse effects, the federal health agencies said.
“It is the job of public health officials around the country to protect the lives of the populations they serve, particularly the vulnerable. Fueling vaccine hesitancy undermines this effort,” said the letter signed by FDA Commissioner Robert Califf and CDC Director Rochelle Walensky.
The Florida Department of Health on Saturday didn’t respond to an email inquiry about the letter.
#us politics#news#ap news#the associated press#2023#florida#covidー19#covid vaccine#coronavirus pandemic#coronavirus vaccine#Food and Drug Administration#Centers for Disease Control and Prevention#Joseph Ladapo#Gov. Ron DeSantis#Florida Department of Health#Rochelle Walensky#Robert Califf
5 notes
·
View notes
Text
FDA Registration Duns Number
If you did not obtain a DUNS Number, your U.S. FDA Registration may have been canceled. Your company risks cargo detentions, Import Alerts and Warning Letters.
ITB HOLDINGS LLC
390 North Orange Avenue, Suite 2300
Orlando, FL 32801
United States
T: +1 855 389 7344
T: +1 855 510 2240
T: +44 800 610 1577
https://www.fda.itbhdg.com/services/

2 notes
·
View notes
Text
I dont know if anyone else has seen the post in question but um The honey that the fda is sending warning letters for having undeclared viagra in it isn’t the normal honey that you’d get in the normal store. Because if you look at the list of products being investigated they are called things like this

So umm. If you just bought normal honey from the grocery store to eat I think you will be fine, actually
10 notes
·
View notes