#Moderna COVID-19 bivalent booster
Text
If you live in the U.S. and you’re 12+, you’re eligible for the updated bivalent anti-omicron booster shot, and it should be available in your local pharmacy.
Please reblog this post, as there’s been very little news coverage and shockingly little propagation of information about the availability of updated boosters.
#covid#COVID-19#boosters#booster shot#covid19#covid vaccine#Omicron#covid booster#bivalent vaccine#cdc#public health#epidemic#vaccine#vaccines#united states#moderna#pfizer#stay safe#vaccine booster#sars-cov-2#shot
35K notes
·
View notes
Text
ACDH to host drive-thru COVID-19 booster clinic Thursday - 9/22/2022
New Post has been published on https://aroundfortwayne.com/news/2022/09/15/acdh-to-host-drive-thru-covid-19-booster-clinic-thursday-20220922/
ACDH to host drive-thru COVID-19 booster clinic Thursday - 9/22/2022

A drive-through vaccination clinic next Thursday, Sept. 22, 2022, will provide Allen County residents with free, newly available COVID-19 vaccinations.
#4813 New Haven Avenue#ACDH Administrator Mindy Waldron#ACDH Allen County Department of Health#ACDH COVID-19 Vaccination Clinic#ACDH Medical Annex#Allen County Health Commissioner Dr. Thomas Gutwein#CDC Centers for Disease Control and Prevention#COVID-19 pandemic#COVID-19 vaccine boosters#COVID-19 variant: Omicron B.1.1.529#Fort Wayne Indiana#Moderna COVID-19 bivalent booster#monkeypox virus vaccination#Pfizer COVID-19 bivalent booster#Pfizer-BioNTech COVID-19 bivalent booster#SR 930#Washington Boulevard
0 notes
Text
🚨💲80 Shohei Ohtani Angels Jerseys🚨
Available in the following sizes (S, M, L, XL, XXL). Follow the link below to get yours today!
https://marconswarehouse.com/shohei-ohtani-cream-jersey.html
Got Questions? Email us here: [email protected]

#covid#COVID-19#boosters#booster shot#covid19#covid vaccine#Omicron#covid booster#bivalent vaccine#cdc#public health#epidemic#vaccine#vaccines#united states#moderna#pfizer#stay safe#vaccine booster#sars-cov-2#shot
0 notes
Text
Updated vaccines against Covid-19 are coming, just as hospitalizations and deaths due to the virus are steadily ticking up again.
Today, the US Food and Drug Administration authorized new mRNA booster shots from Moderna and Pfizer, and a panel of outside experts that advises the Centers for Disease Control and Prevention voted to recommend the shots to everyone in the United States ages 6 months and older. Once Centers for Disease Control and Prevention director Mandy Cohen signs off on the recommendations and the vaccines are shipped, people can start getting the boosters.
The recommendation is projected to prevent about 400,000 hospitalizations and 40,000 deaths over the next two years, according to data presented at the meeting by CDC epidemiologist Megan Wallace.
This year’s mRNA vaccines are different from the 2022 booster in a key way. Last year’s shot was a bivalent vaccine, meaning it covered two variants: the original one that emerged in China in 2019, plus the Omicron subvariant BA.5, which was circulating during much of 2022. This fall’s booster drops the original variant, which is no longer circulating and is unlikely to return. It targets just the Omicron subvariant XBB.1.5, which was dominant throughout much of 2023.
Pfizer and Moderna’s vaccines work by introducing a tiny piece of genetic material called messenger RNA, or mRNA, that carries instructions for making SARS-CoV-2’s characteristic spike protein. Once it is injected, cells in the body use those instructions to temporarily make the spike protein. The immune system recognizes the protein as foreign and generates antibodies against it. Those antibodies stick around so that if they encounter that foreign invader again, they will mount a response against it.
Since the start of the Covid-19 pandemic, the virus has acquired new mutations in its spike protein and elsewhere. These mutations result in new variants and subvariants that diverge from the original virus. When enough mutations accumulate, these new versions can more easily evade the antibodies created by previous vaccine doses or infections.
The constantly evolving nature of the virus is the reason health regulators decided last year to update the original mRNA vaccines, which were designed against the version of the virus that first appeared in 2019. This year, once again, the virus has changed enough to warrant an updated booster.
In June, an advisory committee to the FDA recommended that this fall’s booster be a monovalent vaccine—targeting only the then-dominant XBB.1.5 subvariant.
At that meeting, committee members reviewed evidence suggesting that the inclusion of the original variant may hamper the booster’s effectiveness against newer offshoots. “The previous bivalent vaccine contained the ancestral spike and thus skewed immune responses to the old spike,” says David Ho, a professor of microbiology at Columbia University whose research, which is not yet peer-reviewed, was among the evidence the FDA panel reviewed. “This is what we call immunological imprinting, and it results in lack of immune responses to the new spike.” He thinks taking out the old variant should optimize the immune response.
But over the past few months, even newer Omicron offshoots have arrived. Currently, EG.5.1, or Eris, is the dominant one in the United States, United Kingdom, and China. Meanwhile, a variant called BA.2.86, or Pirola, has been detected in several countries. Pirola has raised alarm bells because it has more than 30 new mutations compared to XBB.1.5.
Even though the new boosters were formulated against XBB.1.5, they’re still expected to provide protection against these new variants. “The reason is, while antibodies are important in protection against mild disease, the critical part of the immune response that’s important for protecting against severe disease is T cells,” says Paul Offit, a professor of vaccinology at the University of Pennsylvania and member of the FDA’s vaccine advisory committee.
These cells are a different part of the immune response. Unlike antibodies, which neutralize a pathogen by preventing it from infecting cells, T cells work by eliminating the cells that have already been invaded and boosting creation of more antibodies. Both the Moderna and Pfizer-BioNTech Covid vaccines produce long-lasting T cells in addition to antibodies.
It’s why, Offit says, when the Omicron wave hit in late 2021 and peaked in January 2022, the US didn’t see a dramatic increase in hospitalizations and deaths even as cases rose significantly: People’s T cells kicked into gear, even when their antibodies didn’t recognize the Omicron variant.
“In some ways,” says Offit, when it comes to vaccine booster development, “it almost doesn’t matter what we pick to target” because the coronavirus has yet to evolve away from T cell recognition. “Everything works.”
Scientists think T cells are able to protect against severe Covid because they’re recognizing parts of the virus that have remained unchanged throughout the pandemic. “I suspect that as we continue to vaccinate, there are some conserved regions [of the virus],” says Jacqueline Miller, Moderna’s head of infectious diseases. “So even with the accumulation of mutations, we’re still building on previous immunity.”
People who have hybrid immunity—that is, have had a Covid infection and have also been vaccinated—seem to have the best immune responses to new variants, she says, which suggests that previous exposure shapes and improves immune responses to new variants. Preliminary studies show that antibodies generated by previous infections and vaccinations should be capable of neutralizing Pirola.
Earlier this month, Moderna issued a press release saying that clinical trial data showed that its updated booster generated a strong immune response against Pirola, as well as the more prevalent Eris variant.
In a statement to WIRED, Pfizer spokesperson Jerica Pitts said the company continues to closely monitor emerging variants and conduct tests of its updated monovalent booster against them. Data presented at Tuesday’s CDC meeting showed that Pfizer-BioNTech’s updated booster elicited a strong neutralizing antibody response against both Eris and Pirola.
The FDA expects that Covid-19 vaccines will continue to be updated on an annual basis, unless a completely new variant emerges that requires a different approach. “We will always be a little behind the virus,” says Ho. “In this instance, we won’t suffer too much, but that might not be the case going forward. Surveillance is imperative.”
814 notes
·
View notes
Text
Brazilian-developed vaccine against Covid-19 registered by Anvisa

The new vaccine against Covid-19 developed by the Brazilian company Zalika Farmacêutica has been entered into the National Health Surveillance Agency (Anvisa) this week, Agencia Brasil reported. The drug can be used in people aged 12 and over and is to be administered in two doses, 21 days apart, with boosters after 6 months for those over 18 years of age.
The technology used in the Zalika vaccine is called “recombinant” because its molecules are formed by combining two different sources. In this case, the protein S antigen (spike) -capable of promoting a response from the immune system- and the saponin-based adjuvant allow the mixture to enhance the production of antibodies. This form of production brings greater safety to the pharmaceutical industry, Anvisa explained in a statement.
The new immunizer is the sixth to receive definitive individual registration from Anvisa, in addition to Comirnaty Ipfizer/Wyeth, Comirnaty bivalent (Pfizer), Janssen Vaccine (Janssen-Cila), Oxford/Covishield (Fiocruz and Astra-Zeneca) and Spikevax bivalent vaccines have received this type of authorization. Pfizer/Biontech, Astra-Zeneca, Janssen, Moderna, Sinopharm, and Sinovac also have definitive registration in the form of the Covax Facility consortium.
Continue reading.
#brazil#brazilian politics#politics#coronavirus#covid 19#vaccination#mod nise da silveira#image description in alt
9 notes
·
View notes
Text
The FDA recommended an additional bivalent booster for:
People age 65 or older who have received one bivalent dose, as long as it is 4 months after that initial shot
Most unvaccinated people, who may now receive a single dose of the bivalent vaccine, rather than multiple doses of the original monovalent mRNA vaccines
Most people who have received a monovalent shot
People who are immunocompromised and have received one bivalent shot. Another booster can be given 2 months later, and more doses may be given at the discretion of, and at times determined by, their health care provider.
For immunocompromised people ages 6 months to 4 years, eligibility will depend on the vaccine they got before.
Children 6 months through 5 years of age who have been vaccinated, but the number of boosters they receive depends on previous vaccines given
The FDA said that unvaccinated children 6 months to 5 years of age can receive a two-dose series of the Moderna bivalent vaccine; or they can get a three-dose series of the Pfizer-BioNTech bivalent vaccine (through age 4). Unvaccinated children who are 5 years old can get two Moderna bivalent boosters or a single Pfizer booster.
20 notes
·
View notes
Text
June 12 (Reuters) - COVID-19 vaccines being developed and manufactured for the 2023-2024 campaign should target one of the currently dominant XBB variants, the U.S. Food and Drug Administration's (FDA) staff reviewers said on Monday.
The comments were made in documents posted ahead of Thursday's meeting of a panel of FDA's independent experts, who are expected to make recommendations on what strain an updated COVID-19 booster should target.
An advisory group to the World Health Organization (WHO) in May recommended that COVID-19 booster shots for the year should be updated to target XBB subvariants.
Last year's COVID vaccine boosters in the United States featured both the original strain of the vaccine and Omicron in a so-called bivalent shot.
About 17% of people in the United States received a COVID booster shot in the 2022-2023 vaccination season, according to CDC data that was current through early May.
COVID-related deaths in the United States spiked in January, but have mostly fallen since then. They fell 14.3% in the past week.
Regulators say vaccines need to be updated to deal with the unpredictability of the virus.
"There is no indication that SARS-CoV-2 evolution is slowing down, though immunity appears to be mitigating severe clinical outcomes," the FDA's staff said.
The COVID vaccination campaign should feature a monovalent vaccine targeting either the XBB.1.5, XBB.1.16, or XBB.2.3, the FDA's reviewers said.
XBB subvariants accounted for more than 95% of the circulating virus variants in the United States by early June 2023, they added.
COVID-19 vaccine makers like Pfizer/BioNtech (PFE.N), Moderna Inc (MRNA.O) and Novavax Inc (NVAX.O) are already developing versions of their respective vaccines targeting XBB.1.5 and other currently circulating strains.
Reporting by Manas Mishra and Bhanvi Satija in Bengaluru and Michael Erman in New York; Editing by Anil D'Silva
#article#COVID shots should target XBB variants in 2023-24 campaign#US FDA staff say#covid 19#COVID-19#COVID#the pandemic
9 notes
·
View notes
Text
These vaccines are the first that aren't rolled out by the U.S. government, and without funding that was directed to public health programs in the state of emergency, the outreach is nowhere near what it was at the height of the pandemic, said Lori T. Freeman, the CEO of the National Association of County and City Health Officials (NACCHO).
. . .
All insurers are legally required to cover the COVID-19 vaccine, and the federal government is stepping in to pay for vaccines for those who lack insurance through the Bridge Access Program. But insurers have been slow to implement these vaccines into their systems, leading to the stuttered rollout of the vaccine, said Dr. William Schaffner, an infectious disease and health policy professor at Vanderbilt University Medical Center
"There have already been people who have gone to their pharmacies and physician's offices looking for the vaccine and have discovered that they haven't been covered yet, so that means they're going to have to come back again," Schaffner told Salon in a phone interview. "A vaccine deferred is often a vaccine that is never received, unfortunately."
. . .
Nationally, COVID hospitalizations have been steadily increasing since June, along with the rise of Omicron variants like EG.5 (nicknamed "Eris") and FL.1.5.1 (nicknamed Fornax.) The vaccines are predicted to work against these strains of the SARS-CoV-2 virus, which evolves naturally in ways that will sometimes render vaccines next to useless. This is why new shots must be developed with some regularity.
. . .
Meanwhile, approximately 18 million Americans have developed long COVID and data suggests that number will continue to rise with more infections. Although the immunocompromised, elderly and people with other health conditions are the most vulnerable to severe infection, COVID-19 continues to be one of the top 10 leading causes of death for children in the U.S.
This rollout, including mRNA vaccines from Pfizer and Moderna, boosts immunity toward Omicron variants. The Centers for Disease Control and Prevention (CDC) recommended the shots for everyone 6 months and up and projects that this could prevent 400,000 hospitalizations and 40,000 deaths over the next two years.
. . .
Regardless, the question remains about how many people will take the new vaccines. Only about one in five people got last year's bivalent booster and one in four adults in the U.S. are completely unvaccinated, according to CDC data and the KFF survey. Although it has been improving over time, uptake has been particularly low in Black communities, in part because vaccination sites are disproportionately located in white neighborhoods but also because of decades of mistrust built up in response to prior medical malpractice.
Notably, just 6 million doses have been put aside for the uninsured through the Bridge Access Program, when at least 27 million people in the U.S. are uninsured, Freeman said. The demand for vaccines is a moving target that distributors are trying to balance without losing money, she added, especially because these vaccines have to be kept cold and take resources to store and administer.
-----
Absolutely get the vaccine! I recommend calling your insurance and pharmacy first though.
4 notes
·
View notes
Text
It took almost 3 years, but I contracted COVID-19 this weekend. My boss appeared sick on Friday, so I gave him a test and he stayed in his office and we were masked and distanced if discussing anything. Woke up Saturday morning with a dry throat. 🥺 By that afternoon, had a very low grade fever of 99°F. Woke up congested on Sunday and tested positive. I took an antihistamine to dry up my mucous, Sinex spray and Sudafed to open up my sinuses. Fever increased to 101.7°F but I didn’t take any analgesics or cough syrup until just before bedtime. I’m coughing and congested again this morning, so same medications were taken this morning. Fever back down to 99°F, so no Tylenol yet. I’m pleased that the illness is this mild. I’m fully supportive of booster vaccines. I got Moderna X 2 in January/February of 2021 and a Pfizer booster in December 2021. My last booster was the bivalent Pfizer in December 2022. I don’t go to many crowded places, but I don’t wear masks 100% of the time either (like in the office). From recent print news, “People who haven't had COVID will likely catch XBB.1.5 – and many will get reinfected, experts say variant XBB.1.5 is very contagious, meaning everyone is at risk even if you've already been infected.” Sounds about right. Also, my boss was sick with COVID last January, and he’s faring better this time around. He’s also vaxxed to the max. Get vaccinated folks—for COVID, for influenza. It *will* keep you out of the hospitals, which are packed this time of year. Much love to everyone out there. Oh, and I plan to use that “COVID brain” excuse to the max, so be prepared. 😏
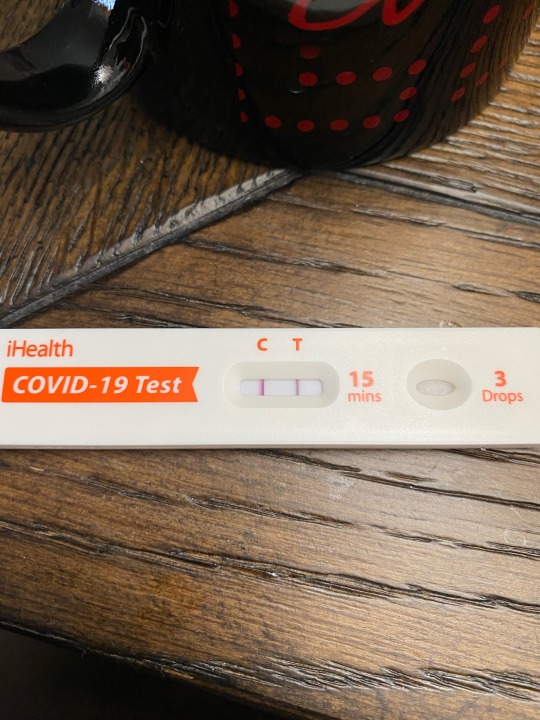
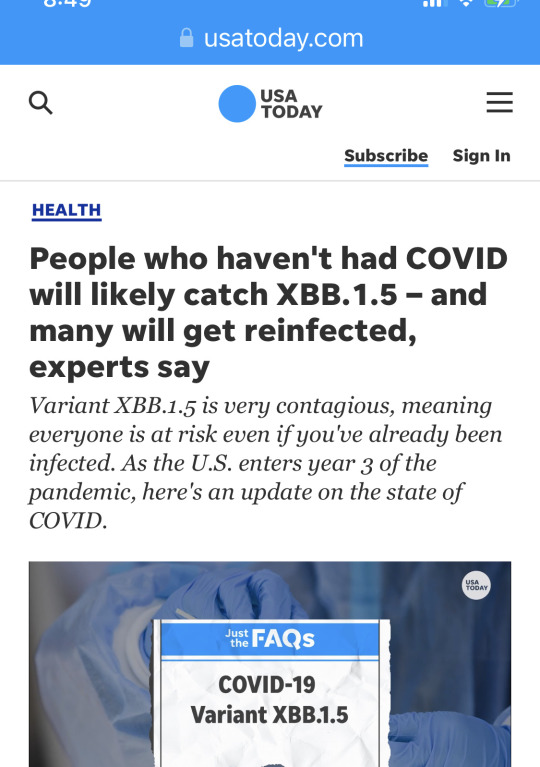
9 notes
·
View notes
Text
Updated bivalent booster vaccines have been authorized and recommended by the FDA and CDC,
respectively, for use in persons 12 years of age or older (Pfizer-BioNTech) or 18 years of age or
older (Moderna) who have completed COVID-19 primary series vaccination.
o Bivalent booster vaccines contain spike protein mRNA for the original (ancestral) and
Omicron BA.4/BA.5 SARS-CoV-2 strains.
o All persons 12 years of age or older who have completed at least a COVID-19 vaccine
primary series should receive a single bivalent vaccine booster (including persons who
received any monovalent boosters after primary series vaccination).
[So I want to and need to get this booster!]
Bivalent vaccine can only be used for booster doses and NOT primary series
vaccination.
o CDC recommends the updated bivalent vaccine boosters be administered at least 2 months
after completion of the primary series (for people who have not received any prior booster
doses), or at least 2 months after the last monovalent booster dose.
NH DPHS suggests waiting at least 3 months after prior vaccination or SARs-CoV-2
infection before administering a bivalent vaccine booster to maximize immune
protection and minimize side effects, but decision should be based on a person’s
COVID-19 risk and immunocompromised status.
o Bivalent boosters have shown superior immunogenicity against Omicron variants with higher
neutralizing antibody titers and likely broader protection against future variants. No new
safety concerns or serious side effects have been identified with bivalent vaccines.
o Review CDC’s updated Interim Clinical Considerations for Use of COVID-19 Vaccines.
Vaccine schedules are different for persons who are moderately or severely
immunocompromised and for persons who are NOT immunocompromised.
o Review FDA’s new Fact Sheet for Healthcare Providers for the updated Pfizer-BioNTech and
Moderna bivalent vaccine boosters.
Do NOT dilute the Pfizer-BioNTech or Moderna bivalent booster vials prior to use.
o Use the FDA’s updated Fact Sheet for Recipients and Caregivers for the Pfizer-BioNTech
and Moderna bivalent vaccine boosters.
Original monovalent mRNA vaccines are no longer able to be used as booster doses in persons 12 years of age or older.
o Monovalent vaccines should continue to be used for primary series vaccination and booster vaccination in children 5-11 years of age who qualify.
o Bivalent vaccine boosters are expected to be extended to younger age groups in the coming weeks.
• Bivalent booster vaccine locations can now be searched on Vaccines.gov.
• Consider administering Evusheld* every 6 months to persons who are moderately or severely immunocompromised to supplement vaccine protection.
• CDC will conduct a provider webinar on Tuesday, 9/13 from 2:00 – 3:00 pm on “Recommendations
for Bivalent COVID-19 Booster Doses in People Ages 12 Years and Older” which can be accessed
at: https://emergency.cdc.gov/coca/calls/2022/callinfo_091322.asp.
• Join our monthly NH Division of Public Health Services (DPHS) healthcare provider webinar for
further updates on bivalent COVID-19 boosters on Thursday, 9/8 from 12:00 – 1:00 pm:
o Zoom link: https://nh-dhhs.zoom.us/s/94059287404
o Call-in phone number: (646) 558-8656
o Meeting ID: 940 5928 7404
o Password: 353809
*EVUSHELD
is an investigational medicine used in adults and adolescents (12 years of age and older who weigh at least 88 pounds [40 kg]) for pre-exposure prophylaxis for prevention of COVID-19 in persons who are:
Not currently infected with SARS-CoV-2 and who have not had recent known close contact with someone who is infected with SARS-CoV-2 and
Who have moderate to severe immune compromise due to a medical condition or have received immunosuppressive medicines or treatments and may not mount an adequate immune response to COVID-19 vaccination or
For whom vaccination with any available COVID-19 vaccine, according to the approved or authorized schedule, is not recommended due to a history of severe adverse reaction to a COVID-19 vaccine(s) or COVID-19 vaccine ingredient(s).
4 notes
·
View notes
Text
2 notes
·
View notes
Text
ISDH: List of locations for new COVID-19 boosters available
New Post has been published on https://aroundfortwayne.com/news/2022/09/13/isdh-list-of-locations-for-new-covid-19-boosters-available/
ISDH: List of locations for new COVID-19 boosters available

Today, the Indiana State Department of Health announced that it has added locations that are offering the new bivalent COVID-19 booster vaccines to its map.
#CDC Centers for Disease Control and Prevention#COVID-19 pandemic#COVID-19 vaccine#COVID-19 vaccine boosters#COVID-19 variant: Delta B.1.617.2#COVID-19 variant: Omicron B.1.1.529#FDA U.S. Food and Drug Administration#Indiana Health Commissioner Dr. Kris Box#Indianapolis Indiana#ISDH Indiana State Department of Health#Moderna COVID-19 bivalent booster#Moderna COVID-19 Vaccine#Pfizer COVID-19 bivalent booster#Pfizer-BioNTech COVID-19 vaccine
0 notes
Text
MMWR Booster #29: Interim Recommendations from the Advisory Committee on Immunization Practices for the Use of Bivalent Booster Doses of COVID-19 Vaccines — United States, October 2022
Top 5 Takeaways
Bivalent Booster Recommendation: In fall 2022, the CDC recommended a bivalent mRNA COVID-19 vaccine booster dose for individuals aged 5 years and older, to be administered at least 2 months after completing the primary series or after receiving a monovalent booster dose.
Booster Dose Composition: Pfizer-BioNTech and Moderna bivalent booster vaccines contain equal amounts of spike mRNA from the ancestral and Omicron BA.4/BA.5 strains, designed to improve protection against these sublineages.
Vaccine Effectiveness Decline: Monovalent COVID-19 vaccines showed decreased effectiveness over time, particularly against the Omicron variant. A third monovalent booster dose provided increased protection, but its effectiveness waned over time, especially against recent Omicron sublineages.
Clinical Trials Data: Clinical trials for Moderna and Pfizer-BioNTech bivalent vaccines demonstrated that they meet superiority criteria for Omicron antibodies, with similar or lower rates of adverse events compared to monovalent boosters.
Equity and Global Access: The ACIP emphasized the importance of equitable COVID-19 vaccination coverage and global access to vaccines, including for younger age groups and minority groups disproportionately affected by COVID-19.
Full summary link: BroadlyEpi.com
Enjoying these summaries? Check back every day at 8am and 4pm Pacific Time (UTC - 8) for a new MMWR Booster. A reblog would also be greatly appreciated, and thanks to everyone who already has! BroadlyEpi hopes to make Epidemiology and Public Health more approachable to anyone who's interested.
0 notes
Text
📆 12 Oct 2023 📰 It’s good to feel bad after your COVID shot 🗞 National Geographic
Jeremy Warner has had six shots of the COVID vaccines. He’s an oncologist at Brown University in Providence, Rhode Island, where he treats immunocompromised patients with cancer who are especially vulnerable to COVID-19. To keep his patients safe, Warner rolls up his sleeves as soon as the U.S. Food and Drug Administration recommends a new vaccine, but he dreads the aftermath. "Each time I'm like, oh my God, I can't do this again!"
After each COVID shot Warner gets a fever, headaches, shaking chills, and painful, swollen joints along with the expected tenderness at the site of injection. "The worst was the second shot that lasted two or three days," recalls Warner. "This recent round, maybe one or two days."
The good news: new research shows that more side effects might be beneficial because they reflect greater production of virus-fighting antibodies after vaccination.

Fear of reactions make some people hesitant to get a COVID shot and a third of adults in one study blamed vaccine side effects for not taking the bivalent boosters last winter that targeted two strains—the original SARS-CoV-2 virus and an Omicron subvariant.
"[But] the side effects show the vaccine is working," says Drew Weissman, an immunologist at the University of Pennsylvania, whose research led the development of the mRNA vaccines, including Moderna's and Pfizer's. Weissman and Katalin Karikó were recently awarded the 2023 Nobel Prize in Physiology or Medicine for their work on modifying mRNA.
COVID-19 vaccines are overwhelmingly safe and effective. Fewer than 1% of the 10.1 million U.S. respondents who have completed health surveys through the Centers for Disease Control's V-safe program—launched in December 2020—have reported needing a medical care after vaccination.
But mRNA vaccines are among the most painful of vaccines, comparable to the shingles vaccine. Scientists don't yet know why. "There's still a lot that's being learned about the side effects of mRNA vaccines," says Deborah Fuller, a vaccinologist at University of Washington School of Medicine, Seattle.
0 notes
Text
All Infected in COVID Outbreak at CDC Conference Were Vaccinated, Agency Confirms
Bivalent Protection
The CDC said the survey results “underline the importance of vaccination for protecting individuals against severe illness and death related to COVID-19” because none of the people who said they tested positive reported going to a hospital.
No clinical trial efficacy data are available for the bivalent shots, even though they were first cleared nine months ago. They provide little protection against infection, according to observational data, though officials maintain they protect against severe illness. That protection is short-lived, according to studies, including non-peer-reviewed CDC publications.
The most recent publication, released on May 26, showed poor effectiveness against hospitalization from the Pfizer and Moderna bivalent COVID-19 vaccines, which replaced the old vaccines earlier this year.
Among adults without “documented immunocompromising conditions,” the protection was 62 percent between seven and 59 days but went to 47 percent before plunging to just 24 percent after 120 days.
Among adults with “documented immunocompromising conditions,” the effectiveness peaked at just 41 percent, hitting 13 percent after 120 days.
Researchers did not provide the effectiveness estimates among all adults, or the combined population of those with and without “documented immunocompromising conditions.” They also did not provide the unadjusted vaccine effectiveness (VE) estimates, or estimates before adjusting for certain variables.
“Both the crude VE and adjusted VE should be reported so that big discrepancies are evident to the reader and questioned,” David Wiseman, founder and president of Synechion, told The Epoch Times via email.
Effective against critical illness—defined as admission to intensive care, or death—peaked at 85 among the people deemed immunocompetent, but plunged to 33 percent after 120 days. Among those described as immunocompromised, the effectiveness was not estimated above 53 percent.
Effectiveness was not measured beyond 180 days.
Effectiveness for children was not examined as part of the research.
CDC researchers looked at data from its VISION Network, a network of hospitals in the United States. Exclusions included people under 50 who received four or more old vaccine boosters.
Just 23.5 percent of the immunocompetent and 16.4 percent of the immunocompromised were vaccinated, while the rest had received at least two doses of a COVID-19 vaccine.
About 8 percent of American adults are still unvaccinated, according to CDC data, though that percentage may be a big overestimate (pdf).
Researchers said the data showed that bivalent doses “helped provide protection against COVID-19-associated hospitalization and critical disease” adding that “waning of protection was evidence in some groups.”
0 notes
Text
All Infected in COVID Outbreak at CDC Conference Were Vaccinated, Agency Confirms
Bivalent Protection
The CDC said the survey results “underline the importance of vaccination for protecting individuals against severe illness and death related to COVID-19” because none of the people who said they tested positive reported going to a hospital.
No clinical trial efficacy data are available for the bivalent shots, even though they were first cleared nine months ago. They provide little protection against infection, according to observational data, though officials maintain they protect against severe illness. That protection is short-lived, according to studies, including non-peer-reviewed CDC publications.
The most recent publication, released on May 26, showed poor effectiveness against hospitalization from the Pfizer and Moderna bivalent COVID-19 vaccines, which replaced the old vaccines earlier this year.
Among adults without “documented immunocompromising conditions,” the protection was 62 percent between seven and 59 days but went to 47 percent before plunging to just 24 percent after 120 days.
Among adults with “documented immunocompromising conditions,” the effectiveness peaked at just 41 percent, hitting 13 percent after 120 days.
Researchers did not provide the effectiveness estimates among all adults, or the combined population of those with and without “documented immunocompromising conditions.” They also did not provide the unadjusted vaccine effectiveness (VE) estimates, or estimates before adjusting for certain variables.
“Both the crude VE and adjusted VE should be reported so that big discrepancies are evident to the reader and questioned,” David Wiseman, founder and president of Synechion, told The Epoch Times via email.
Effective against critical illness—defined as admission to intensive care, or death—peaked at 85 among the people deemed immunocompetent, but plunged to 33 percent after 120 days. Among those described as immunocompromised, the effectiveness was not estimated above 53 percent.
Effectiveness was not measured beyond 180 days.
Effectiveness for children was not examined as part of the research.
CDC researchers looked at data from its VISION Network, a network of hospitals in the United States. Exclusions included people under 50 who received four or more old vaccine boosters.
Just 23.5 percent of the immunocompetent and 16.4 percent of the immunocompromised were vaccinated, while the rest had received at least two doses of a COVID-19 vaccine.
About 8 percent of American adults are still unvaccinated, according to CDC data, though that percentage may be a big overestimate (pdf).
Researchers said the data showed that bivalent doses “helped provide protection against COVID-19-associated hospitalization and critical disease” adding that “waning of protection was evidence in some groups.”
0 notes