#fwater
Text
augh the agony
*dtrijks a bottle of fwater*
woaga... im so normal now
1 note
·
View note
Text
the precision of an overlit pussy is nonexistent
red light green light
where's her pot to piss in
if she's open all. the time. the security is lost
no bouncer. to be. seen.
the court of a queen
gone. to the races they say, but its disputed.
the pain of a prophet with his memories refuted to the time that says otherwise and drinks his blood with glee.
ou revoir
says he
to the court and to the queen
now she's hardened as a bean before. sprouting
before tip the touch o fwater its astounding
can I take a moment to marvel in the defenses of her queendom?
0 notes
Text
Aquasana AQ 5300 Review (Updated: [year])

Debating whether or not you should buy the Aquasana AQ 5300? Wondering how this under-sink water filter system compares to its top competitors?
In this review, we've shared everything you need to know about the features, specs, performance, and pros and cons of the Aquasana AQ 5300.
🧾 Overview
The Aquasana AQ 5300, also called the Aquasana 3 Stage Max Flow Claryum Under Sink Water Filter System, is a multi-stage under-sink water filtration system that is NSF tested and certified to remove 77 contaminants from drinking water.
This compact filtration system comes with a dedicated faucet and has a 4-6-month filter lifespan.
The AQ 5300 is Aquasana's mid-level under-sink water filter system model. Aquasana also offers a smaller two-stage system (the Aquasana AQ-5200) and a 3-stage filter that's exactly the same as the AQ 5300 but has larger filter cartridges and a bigger capacity of 800 gallons (the AQ 5300+).
4/5★★★★★
📝 System Specifications
Filter TypeUnder-sinkFilter Stages3Filter Materials20-micron sediment filter; activated and catalytic carbon media; ion-exchange resinOperating Pressure20-80 psiOperating Temperature40-90°FWater Flow Rate (GPM)0.5Maximum Capacity600 gallons/ 4-6 monthsSystem Dimensions (L x W x H)4.25 x 12 x 12.85 inchesWarranty & Guarantee1-year warranty, 90-day guarantee
💡 Features
BPA-Free Design
The Aquasana AQ 5300 is made from 100% BPA-free plastic. That means you can enjoy your filtered tap water without worrying about added nasties.
3-Stage Filtration
There are three stages of filtration involved in this water filter system: a sediment filter, and two catalytic carbon and ion exchange filters.
Removes 77 Contaminants
This Aquasana model is NSF certified to remove 77 contaminants, including lead, cysts, chlorine, mercury, and asbestos.
3 Faucet Materials
You can choose between three materials for the included faucet: brushed nickel, chrome, and oil-rubbed bronze. Different styles and colors suit different kitchens.
1-Year Warranty
The AQ 5300 is backed by a 1-year warranty and comes with a 90-day money-back guarantee, so you can buy this system risk-free.
🚦 Performance
We measure the performance of under-sink water filters by testing and assessing three factors:
- Flow rate and speed of water delivery
- Effectiveness of the filtration process and contaminants removed
- Longevity - how long the system retains optimal performance
We rated the performance effectiveness of the Aquasana 3 Stage Max Flow filter highly. The filter removes a whole host of contaminants and transforms ordinary tap water into clean, fresh drinking water. We noticed an immediate improvement in the taste and quality of our water after installing this filter.
The filter only removes the bad stuff from your water, too. Retaining natural beneficial minerals like calcium and potassium maintains a pleasant alkaline taste in water. No chemicals or plastics are added to the water from the filter media and casing (as you would hope), so the system doesn't introduce anything dangerous or unhealthy to your drinking water.
Plus, this Aquasana model is certified to NSF Standards 42, 53, and 401 for its performance, which is hugely reassuring. An NSF certification tells you that a water filter is capable of performing according to the manufacturer's claims. NSF 42 is for chlorine; 53 is for contaminants with health effects; and 401 is for incidental contaminants and emerging compounds (like pharmaceuticals).
Looking at flow rate, the Aquasana 5300 delivers a healthy water flow of 0.5 gallons per minute (GPM). The average flow rate for a kitchen faucet is between 1.0 GPM and 2.2 GPM, so it'll take slightly longer than normal to fill a glass of water after installing this filter.
In our testing, the flow rate from the included faucet was steady and nothing to complain about. You still get access to filtered water much faster than traditional drip filters - but keep in mind that you won't get a powerful spray from your faucet, especially when the filters are nearing the end of their lifespans.
The Aquasana 5300 has a 600-gallon capacity, equating to 4-6 months, which is slightly below average for an under-sink filtration system. Most under-sink units have filters that last for 6, 9, or 12 months depending on the filter type.
🔧 Installation & Maintenance
The Aquasana AQ 5300 comes with everything needed to install the system underneath your kitchen sink, including;
- User manual
- Dedicated faucet
- Faucet rubber washer
- Faucet spacer
- Faucet nut
- 3 filter cartridges
- Poly tubing
- Screws with anchors
- Chrome nut
- Plastic tube insert
- Plastic collar
- Brass tube insert
- Brass nut
- 3/8″ inch brass tee
If you're a handy person or you can confidently follow instructions, you should be able to install the Aquasana AQ 5300 without professional help. The installation manual is clear, with a simple step-by-step process to follow. Most people should be able to install this filtration system within one hour.
Visual learners can use Aquasana's instruction video to install the 3-Stage Max Flow Claryum Under Sink Water Filter.
https://www.youtube.com/watch?v=00II7DPxE9Y&ab_channel=aquasana
As for maintenance, your main job is to change the filters once they reach their maximum capacity (usually every 4-6 months). Changing the filters is as simple as twisting off the housing, swapping out the filter, and twisting the housing back in place. There are no hoses or water lines to disconnect.
The unit has a battery alarm that will flash red when it's time to replace your filters. Replacement filter cartridges come with a new battery, so you'll never lose charge.
You can save money on replacement filters by signing up for Aquasana’s Water for Life program.
📝 Filter Info
The Aquasana AQ 5300 uses Aquasana's claryum filtration technology to remove 77 common drinking water contaminants. There are three filter stages involved in this system:
- A 20-micron sediment pre-filter, which removes large particles of sediment and rust, and protects the later filter stages from contaminant damage.
- Two stages of activated carbon and ion exchange resin, which use adsorption to trap contaminants in their media.
We were pleased with the filter's contaminant removal abilities in our testing. This filtration system doesn't only remove the most common easy-remove contaminants (like chlorine and lead) - it also removes:
- Cysts
- PFOA/PFOS
- Pesticides
- Mercury
- Asbestos
- Pharmaceuticals
The filter achieves a similar purity to reverse osmosis water, but with a notable difference: Aquasana's water filters don't remove healthy minerals from water. So, with this model, you get the best of both worlds. You can view a complete list of all the contaminants removed by the AQ 5300 on this datasheet.
🔔 Pros & Cons
👍 What We Like
- We appreciate that the Aquasana AQ 5300 retains healthy minerals in water, so it only gets rid of the bad stuff. That's ideal if you want to drink healthier water without the impurities.
- This under-sink water filter system delivers great-tasting water in a matter of seconds.
- It's easy to hook this system up to your cold water line following Aquasana's video or user manual instructions.
- There are minimal disposable plastic parts in this filtration system, so it's a top choice if you're looking for an environmentally-friendly water treatment solution.
- The unit is backed by a warranty and a guarantee, so you can try it risk-free.
👎 What We Don’t Like
- This filter isn't designed to completely remove total dissolved solids. If you're looking for the most thorough filtration possible and you don't mind water waste, consider Aquasana's reverse osmosis systems.
- The Aquasana 5300 isn't the most economical filter we've tried. A 6-month lifespan for all filters is slightly lower than average. Many under-sink systems have filters lasting up to 12 months.
- This system isn't intended to be used with an existing faucet - you're supposed to install the included faucet to deliver the filtered water. The quality of the included faucets is poor.
- Multiple customers experienced leaks from the disconnect hoses, tubes, and filter housing.
❔ Frequently Asked Questions
Does Aquasana 5300 remove fluoride?
No, the Aquasana 5300 doesn't remove fluoride. If you want to remove fluoride with an Aquasana product, consider the Aquasana Reverse Osmosis System with Re-mineralizer.
How long does Aquasana system last?
The lifespan of the Aquasana 5300 is 4-6 months. After this, you'll need to replace the three filter cartridges with new filters. The unit itself should last for 5-10 years or even longer with proper cleaning and maintenance.
Do Aquasana filters remove bacteria?
Some of Aquasana's filters, like the Aquasana Rhino Whole House Well Water Filter and the Aquasana Reverse Osmosis System with Re-mineralizer, remove bacteria. Aquasana's under-sink filter range, including the Aquasana 3 stage Max Flow Under Sink Water Filter System, don't remove bacteria.
Are Aquasana filters safe?
Yes, Aquasana filters are safe - as long as you follow the instructions to properly maintain them. Don't use the filter cartridges in the Aquasana AQ 3500 for more than six months to prevent the growth of bacteria. Additionally, the Aquasana AQ 3500 isn't safe for treating well water.
Can I use my own faucet with Aquasana?
Yes, you can use your own faucet with the Aquasana AQ 3500. However, Aquasana recommended using the dedicated faucet to prevent contaminants from your own faucet from leaching into the filtered water.
How much does it cost to install Aquasana?
If you install the Aquasana AQ 5300 yourself, the cost to install the system should be $0. The unit comes with all the parts needed for installation, including three filter cartridges. If you decide to hire somebody to install the system for you, you'll need to pay around $50, depending on the contractors' prices in your area.
Found this review helpful?
Comment below or share this article!
Read the full article
0 notes
Photo

Blurry like my life
#flower#Black and White#b&w#personal#fwater#drop#dew#dew drop#indie#alternative#picture#pohotgraphy#grainy
1 note
·
View note
Note
also, my days suck lately, but fortunately when i see your replies it makes my day and i'm feeling totally better!💞 i just noticed that someone recommended your blog for a hq content. so who's right about that precious hq blog of yours? hm? can't hear ya?? + you answered with a mEME ilysm do it more often plz💞💕💖💘💜💞💕💞💔💘💖 i don't know what can i say about me. i'm complicated, that's all i can say probably hahah have a nice day my dear ily. be safe! - your sv🌙
aww oh no ;;; (icb my soulmate was feeling down and i didn’t know???!? T-T ) pls know that whatever ur dealing w rn ;; it will get better!! even if it might not seem like it~ you can get through this i believe in u and im cheering u on!! (also! do u have any other groups u stan? 👀 )
SGLKJDFOISDJG stOP im blushing omygod ahhdslkgjsoifsdg i actually couldn’t believe my eyes when i saw that notif laskdgjoasidjf honestly aHH like this blog.. is a trashbin tho?? lskdjgosdijf ( @hyuunjins hi allison ty again for including me in ur rec!! ;;;; ahhhlsdkgsjdoij 😭😭❤ )
#replies#sv anon#anonymous#HI U ANGEL#sldgjaoisdf#another ask from sv anon which has left me crying and melting lskdjgosidjfsdg#PLS BE SAFE... i almost fell on ice today lsdkgsdf.. wear warm clothes and drink lots o fwater!!!#ilyyy<33
2 notes
·
View notes
Note
Father, Give Me The Herpes Water
foamf actually stands for
father
of
all
mherpes
fwater
3 notes
·
View notes
Text
i should ‘ve had like 5 pbottles of fwater buti nstad i’m aving one (1 ) moutnain doew
1 note
·
View note
Photo

Hello everyone just sharing a few more pictures from last nights Super Pink Full Moon Casting...✨✨✨✨ 🌟🌟🌟🌟🌟🌟🌟🌟🌟 FREE EMAIL CONSULTATIONS with Salubrious Surrey and Solitaire Bon Lavi 💫 Spellbinding Sisters 💫 💁🏽💁🏼💜🙏🏼✨💀 Voodoo Practitioners in Florida Servicing clients worldwide 🌎 With their Spell Casting needs. Over 30yrs of experience combined. 🙏🏼 💞💞💞💞💞💞💞💞💞 Love Spells, Money and Business Spells, Gris Gris bags, Custom work and Specialty Spells, Voodoo dolls, Love Bindings, End a relationship Hex and much more. www.spellbindingsisters.com Soon we will be adding a few *NEW* Items to our homemade supply list for order. They are in the works as we speak 😍🌱 Are you excited? ▶▶▶▶▶▶▶▶▶▶▶▶▶▶▶ FREE Email consultations [email protected] 🌸🌸🌸🌸🌸🌸🌸🌸 Spiritual supplies and Free shipping within USA on all Supply orders and Spell work that is mailed to you. ♓♒♑♐♏♎♍♌♋♊ Salubrious Surrey and Solitaire Bon Lavi servicing clients WORLDWIDE! Spellbindingsisters.com Be sure to follow us for more great content! @spellbindingsisters 🌞 . . . . . . . . . . . . #Fullmoonrituals #spellwork #voodoopractitioners #marktwain #vessel #poured #lwa #love understanding fwater #scorpio #magickal #honoring #erzuliefreda #erzuliedantor #chanting #fire #sweet #united #witchsalubrious (at Canada,Toronto) https://www.instagram.com/p/COLs7ggnWFJ/?igshid=wecufvxd3pvf
#fullmoonrituals#spellwork#voodoopractitioners#marktwain#vessel#poured#lwa#love#scorpio#magickal#honoring#erzuliefreda#erzuliedantor#chanting#fire#sweet#united#witchsalubrious
0 notes
Text
m-Phenylenediamine CAS#: 108-45-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Namem-PhenylenediamineIUPAC Namebenzene-1,3-diamineMolecular Structure

CAS Registry Number 108-45-2EINECS Number203-584-7MDL NumberMFCD00007799Beilstein Registry Number471357Synonymsm-phenylenediamine, 1,3-phenylenediamineMolecular FormulaC6H8N2Molecular Weight108.143InChIInChI=1S/C6H8N2/c7-5-2-1-3-6(8)4-5/h1-4H,7-8H2InChI KeyWZCQRUWWHSTZEM-UHFFFAOYSA-NCanonical SMILESC1=CC(=CC(=C1)N)N
Patent InformationPatent IDTitlePublication DateCN109134267A synthetic method of the compound aromatic amines (by machine translation) 2019CN109810019A compound and its preparation method and as a fluorescence sensor application (by machine translation) 2019CN108164452A double-(mild imide) preparation method (by machine translation) 2018CN109081793Sulfonamide urease inhibitor and its preparation and use (by machine translation)2018CN109096156Between the two sulfonyl aryl diamine urease inhibitor and its preparation and use (by machine translation)2018WO2017/130103PROCESS FOR PREPARING A TRANSITION METAL-SCHIFF BASE IMINE LIGAND COMPLEX2017CN104058993Photosensitive diamine, polyamide acid or the derivatives thereof, the liquid crystal aligning agent, liquid crystal orientation film and liquid crystal display element (by machine translation)2017
Physical Data
AppearanceColorless needle crystalVapor Density3.7 (vs air)Vapor Pressure0.62 mm Hg ( 100 °C)Flash Point>230 °FWater Solubility350 g/L (25 ºC)Pka5.11, 2.50(at 20℃)
Melting Point, °C Solvent (Melting Point) 65toluene, cyclohexane64 - 67
Boiling Point, °CPressure (Boiling Point), Torr1401014622282 - 284760
Sublimation, °CPressure (Sublimation), Torr100 - 1104
Density, g·cm-3Reference Temperature, °C Measurement Temperature, °C1.05541251.07741001.0844901.138915151.13372525
Refractive IndexWavelength (Refractive Index), nmTemperature (Refractive Index), °C1.62558656.357.71.633958957.71.6761743457.7
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d125600Chemical shifts, Spectrum13Cchloroform-d125150Chemical shifts1Hchloroform-d124.84300Chemical shifts, Spectrum13Cchloroform-d124.8475
m-Phenylenediamine CAS#: 108-45-2 NMR

Description (IR Spectroscopy)Solvent (IR Spectroscopy)Intensity of IR bands, Bands, Spectrumsodium chlorideBandspotassium bromidem-Phenylenediamine CAS#: 108-45-2 IR

m-Phenylenediamine CAS#: 108-45-2 Raman
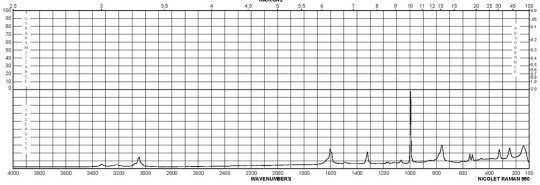
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1acetonitrile215, 29558100, 4900Spectrumethanol200 - 250 nmAbsorption maximaethanol2922300Spectrumethanol250 - 450 nmAbsorption maximaH2O289, 238, 2101995, 7244, 32359Absorption maximamethanol 292.5, 242.5, 213.52291, 7079, 30199
Route of Synthesis (ROS)

Route of Synthesis (ROS) of m-Phenylenediamine CAS 108-45-2
ConditionsYieldWith sodium tetrahydroborate In water at 20℃ for 1h100%With hydrogen; sodium fluoride In methanol at 39.84℃ for 2.17100%With hydrogen In ethanol at 20℃ under 760.051 Torr for 3h chemoselective reaction
Experimental Procedure
General procedure: In this work, the following of nitroaromatic compoundswere used:The liquid-phase hydrogenation reaction of nitroaromaticcompounds can be presented as:It was carried out at room temperature under an atmosphericpressure of H2. A sample of the catalyst(50 mg) was placed in a round-bottomed threeneckedflask and the system was purged with hydrogenfor 30 min. A solution of the substrate in ethanol 0.2 M(or in tetrahydrofuran in the case of using o- andp-dinitrobenzene as a substrate) was then poured intothe reactor by means of a feed cock of the reactionmixture. The concentration of platinum in the reactionmixture was 0.85 mol %. The reaction was carriedout with vigorous stirring on a magnetic stirrer at a rateof 900 rpm in the monitoring mode by means of gasliquidchromatography until the peak of the startingcompound disappeared on the chromatogram. At theend of the experiment, the stirring was stopped; thecatalyst was separated from the reaction mixture bycentrifugation.Analysis of the reaction products was performedusing GC with a Chromatek Crystal 5000.2 chromatographwith a flame ionization detector (FID) and acapillary column CR-5 (2 mm × 25 m) at a temperatureof 190°C. The peaks were identified on the basis of the experimentally obtained retention times of individualcompounds (nitrobenzene, o-dinitrobenzene,p-dinitrobenzene, p-hydroxynitrobenzene, p-nitroacetophenone,nitrocyclohexane, aniline, o-phenylenediamine,p-phenylenediamine, p-hydroxyaniline,p-nitrophenylethanol, aminocyclohexane). Thestructure and purity of the products obtained wereconfirmed by NMR spectroscopy. The 1H and 13CNMR spectra were recorded on a Bruker Avance 300spectrometer in solvents CDCl3 and DMSO-d6.100%With hydrazine hydrate In ethanol at 85℃ for 0.416667h99%With borane-ammonia complex In methanol; water at 20℃ for 0.0833333h99%With ammonia borane; Pd/MIL-101 In methanol; water at 20℃ for 0.05h99%
Safety and Hazards
Pictogram(s)




SignalDangerGHS Hazard StatementsH301: Toxic if swallowed
H311: Toxic in contact with skin
H317: May cause an allergic skin reaction
H319: Causes serious eye irritation
H331: Toxic if inhaled
H341: Suspected of causing genetic defects
H400: Very toxic to aquatic life
H410: Very toxic to aquatic life with long lasting effects
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P311, P312, P321, P322, P330, P333+P313, P337+P313, P361, P363, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationClass 6.1; Packaging Group: III; UN Number: 1673 Under the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD 7.5/kg
Use Pattern m-Phenylenediamine CAS 108-45-2 may be used in the synthesis of the following:
• intrinsically electrically semiconducting microparticles of semiladder poly(m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) structures
• extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite, used as sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls
• series of terpolymers, via chemical oxidative polymerization
• thin film composite (TFC) membranes based polyamide
• TFC reverse osmosis (RO) membranes m-Phenylenediamine (MPD) is an aromatic diamine. It copolymerizes with iso- or terephthaloyl chloride and 3,5-diaminobenzoic acid or 2,4-diaminobenzene sulfonic acid (or sulfonate) to afford copolyamides by low temperature solution polymerization. MPD has been reported to be formed selectively during the hydrogenation of m-dinitrobenzene in the presence of bimetallic Ni-Pt catalysts supported on carbon. Cyclic voltammetric method has been proposed to study the electrochemical copolymerization of aniline withMPD in sulfuric acid solution.
Read the full article
0 notes
Text
#kay what if we call them by their last names?#finnwater?#black...fin...#fwater#binn#winn#blackwinn#pasha#presha#damn their names#aplfm
0 notes
Text
m-Phenylenediamine CAS#: 108-45-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Namem-PhenylenediamineIUPAC Namebenzene-1,3-diamineMolecular Structure

CAS Registry Number 108-45-2EINECS Number203-584-7MDL NumberMFCD00007799Beilstein Registry Number471357Synonymsm-phenylenediamine, 1,3-phenylenediamineMolecular FormulaC6H8N2Molecular Weight108.143InChIInChI=1S/C6H8N2/c7-5-2-1-3-6(8)4-5/h1-4H,7-8H2InChI KeyWZCQRUWWHSTZEM-UHFFFAOYSA-NCanonical SMILESC1=CC(=CC(=C1)N)N
Patent InformationPatent IDTitlePublication DateCN109134267A synthetic method of the compound aromatic amines (by machine translation) 2019CN109810019A compound and its preparation method and as a fluorescence sensor application (by machine translation) 2019CN108164452A double-(mild imide) preparation method (by machine translation) 2018CN109081793Sulfonamide urease inhibitor and its preparation and use (by machine translation)2018CN109096156Between the two sulfonyl aryl diamine urease inhibitor and its preparation and use (by machine translation)2018WO2017/130103PROCESS FOR PREPARING A TRANSITION METAL-SCHIFF BASE IMINE LIGAND COMPLEX2017CN104058993Photosensitive diamine, polyamide acid or the derivatives thereof, the liquid crystal aligning agent, liquid crystal orientation film and liquid crystal display element (by machine translation)2017
Physical Data
AppearanceColorless needle crystalVapor Density3.7 (vs air)Vapor Pressure0.62 mm Hg ( 100 °C)Flash Point>230 °FWater Solubility350 g/L (25 ºC)Pka5.11, 2.50(at 20℃)
Melting Point, °C Solvent (Melting Point) 65toluene, cyclohexane64 - 67
Boiling Point, °CPressure (Boiling Point), Torr1401014622282 - 284760
Sublimation, °CPressure (Sublimation), Torr100 - 1104
Density, g·cm-3Reference Temperature, °C Measurement Temperature, °C1.05541251.07741001.0844901.138915151.13372525
Refractive IndexWavelength (Refractive Index), nmTemperature (Refractive Index), °C1.62558656.357.71.633958957.71.6761743457.7
Spectra
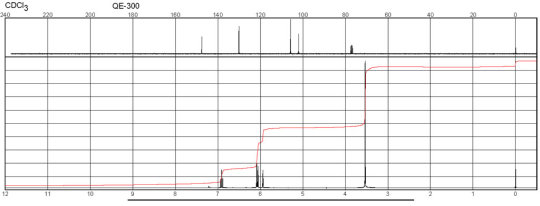
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d125600Chemical shifts, Spectrum13Cchloroform-d125150Chemical shifts1Hchloroform-d124.84300Chemical shifts, Spectrum13Cchloroform-d124.8475
m-Phenylenediamine CAS#: 108-45-2 NMR
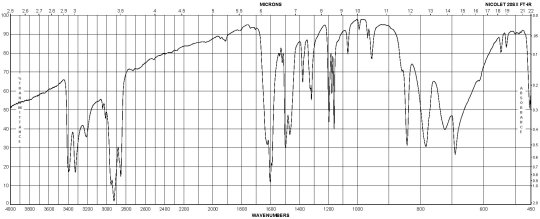
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Intensity of IR bands, Bands, Spectrumsodium chlorideBandspotassium bromidem-Phenylenediamine CAS#: 108-45-2 IR
m-Phenylenediamine CAS#: 108-45-2 Raman
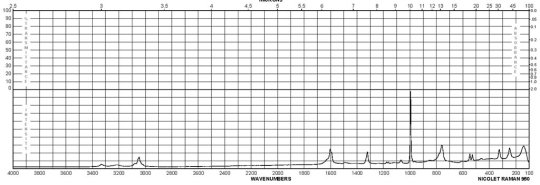
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1acetonitrile215, 29558100, 4900Spectrumethanol200 - 250 nmAbsorption maximaethanol2922300Spectrumethanol250 - 450 nmAbsorption maximaH2O289, 238, 2101995, 7244, 32359Absorption maximamethanol 292.5, 242.5, 213.52291, 7079, 30199
Route of Synthesis (ROS)

Route of Synthesis (ROS) of m-Phenylenediamine CAS 108-45-2
ConditionsYieldWith sodium tetrahydroborate In water at 20℃ for 1h100%With hydrogen; sodium fluoride In methanol at 39.84℃ for 2.17100%With hydrogen In ethanol at 20℃ under 760.051 Torr for 3h chemoselective reaction
Experimental Procedure
General procedure: In this work, the following of nitroaromatic compoundswere used:The liquid-phase hydrogenation reaction of nitroaromaticcompounds can be presented as:It was carried out at room temperature under an atmosphericpressure of H2. A sample of the catalyst(50 mg) was placed in a round-bottomed threeneckedflask and the system was purged with hydrogenfor 30 min. A solution of the substrate in ethanol 0.2 M(or in tetrahydrofuran in the case of using o- andp-dinitrobenzene as a substrate) was then poured intothe reactor by means of a feed cock of the reactionmixture. The concentration of platinum in the reactionmixture was 0.85 mol %. The reaction was carriedout with vigorous stirring on a magnetic stirrer at a rateof 900 rpm in the monitoring mode by means of gasliquidchromatography until the peak of the startingcompound disappeared on the chromatogram. At theend of the experiment, the stirring was stopped; thecatalyst was separated from the reaction mixture bycentrifugation.Analysis of the reaction products was performedusing GC with a Chromatek Crystal 5000.2 chromatographwith a flame ionization detector (FID) and acapillary column CR-5 (2 mm × 25 m) at a temperatureof 190°C. The peaks were identified on the basis of the experimentally obtained retention times of individualcompounds (nitrobenzene, o-dinitrobenzene,p-dinitrobenzene, p-hydroxynitrobenzene, p-nitroacetophenone,nitrocyclohexane, aniline, o-phenylenediamine,p-phenylenediamine, p-hydroxyaniline,p-nitrophenylethanol, aminocyclohexane). Thestructure and purity of the products obtained wereconfirmed by NMR spectroscopy. The 1H and 13CNMR spectra were recorded on a Bruker Avance 300spectrometer in solvents CDCl3 and DMSO-d6.100%With hydrazine hydrate In ethanol at 85℃ for 0.416667h99%With borane-ammonia complex In methanol; water at 20℃ for 0.0833333h99%With ammonia borane; Pd/MIL-101 In methanol; water at 20℃ for 0.05h99%
Safety and Hazards
Pictogram(s)




SignalDangerGHS Hazard StatementsH301: Toxic if swallowed
H311: Toxic in contact with skin
H317: May cause an allergic skin reaction
H319: Causes serious eye irritation
H331: Toxic if inhaled
H341: Suspected of causing genetic defects
H400: Very toxic to aquatic life
H410: Very toxic to aquatic life with long lasting effects
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P311, P312, P321, P322, P330, P333+P313, P337+P313, P361, P363, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationClass 6.1; Packaging Group: III; UN Number: 1673 Under the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD 7.5/kg
Use Pattern m-Phenylenediamine CAS 108-45-2 may be used in the synthesis of the following:
• intrinsically electrically semiconducting microparticles of semiladder poly(m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) structures
• extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite, used as sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls
• series of terpolymers, via chemical oxidative polymerization
• thin film composite (TFC) membranes based polyamide
• TFC reverse osmosis (RO) membranes m-Phenylenediamine (MPD) is an aromatic diamine. It copolymerizes with iso- or terephthaloyl chloride and 3,5-diaminobenzoic acid or 2,4-diaminobenzene sulfonic acid (or sulfonate) to afford copolyamides by low temperature solution polymerization. MPD has been reported to be formed selectively during the hydrogenation of m-dinitrobenzene in the presence of bimetallic Ni-Pt catalysts supported on carbon. Cyclic voltammetric method has been proposed to study the electrochemical copolymerization of aniline withMPD in sulfuric acid solution.
Read the full article
0 notes
Text
m-Phenylenediamine CAS#: 108-45-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Namem-PhenylenediamineIUPAC Namebenzene-1,3-diamineMolecular Structure
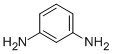
CAS Registry Number 108-45-2EINECS Number203-584-7MDL NumberMFCD00007799Beilstein Registry Number471357Synonymsm-phenylenediamine, 1,3-phenylenediamineMolecular FormulaC6H8N2Molecular Weight108.143InChIInChI=1S/C6H8N2/c7-5-2-1-3-6(8)4-5/h1-4H,7-8H2InChI KeyWZCQRUWWHSTZEM-UHFFFAOYSA-NCanonical SMILESC1=CC(=CC(=C1)N)N
Patent InformationPatent IDTitlePublication DateCN109134267A synthetic method of the compound aromatic amines (by machine translation) 2019CN109810019A compound and its preparation method and as a fluorescence sensor application (by machine translation) 2019CN108164452A double-(mild imide) preparation method (by machine translation) 2018CN109081793Sulfonamide urease inhibitor and its preparation and use (by machine translation)2018CN109096156Between the two sulfonyl aryl diamine urease inhibitor and its preparation and use (by machine translation)2018WO2017/130103PROCESS FOR PREPARING A TRANSITION METAL-SCHIFF BASE IMINE LIGAND COMPLEX2017CN104058993Photosensitive diamine, polyamide acid or the derivatives thereof, the liquid crystal aligning agent, liquid crystal orientation film and liquid crystal display element (by machine translation)2017
Physical Data
AppearanceColorless needle crystalVapor Density3.7 (vs air)Vapor Pressure0.62 mm Hg ( 100 °C)Flash Point>230 °FWater Solubility350 g/L (25 ºC)Pka5.11, 2.50(at 20℃)
Melting Point, °C Solvent (Melting Point) 65toluene, cyclohexane64 - 67
Boiling Point, °CPressure (Boiling Point), Torr1401014622282 - 284760
Sublimation, °CPressure (Sublimation), Torr100 - 1104
Density, g·cm-3Reference Temperature, °C Measurement Temperature, °C1.05541251.07741001.0844901.138915151.13372525
Refractive IndexWavelength (Refractive Index), nmTemperature (Refractive Index), °C1.62558656.357.71.633958957.71.6761743457.7
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d125600Chemical shifts, Spectrum13Cchloroform-d125150Chemical shifts1Hchloroform-d124.84300Chemical shifts, Spectrum13Cchloroform-d124.8475
m-Phenylenediamine CAS#: 108-45-2 NMR
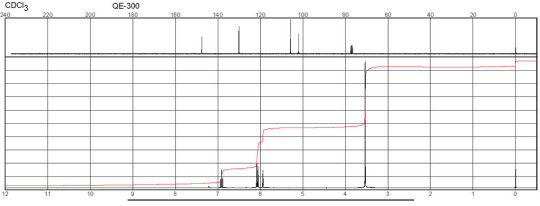
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Intensity of IR bands, Bands, Spectrumsodium chlorideBandspotassium bromidem-Phenylenediamine CAS#: 108-45-2 IR
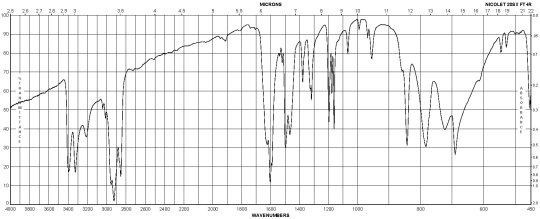
m-Phenylenediamine CAS#: 108-45-2 Raman
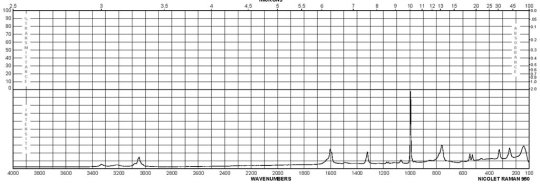
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1acetonitrile215, 29558100, 4900Spectrumethanol200 - 250 nmAbsorption maximaethanol2922300Spectrumethanol250 - 450 nmAbsorption maximaH2O289, 238, 2101995, 7244, 32359Absorption maximamethanol 292.5, 242.5, 213.52291, 7079, 30199
Route of Synthesis (ROS)

Route of Synthesis (ROS) of m-Phenylenediamine CAS 108-45-2
ConditionsYieldWith sodium tetrahydroborate In water at 20℃ for 1h100%With hydrogen; sodium fluoride In methanol at 39.84℃ for 2.17100%With hydrogen In ethanol at 20℃ under 760.051 Torr for 3h chemoselective reaction
Experimental Procedure
General procedure: In this work, the following of nitroaromatic compoundswere used:The liquid-phase hydrogenation reaction of nitroaromaticcompounds can be presented as:It was carried out at room temperature under an atmosphericpressure of H2. A sample of the catalyst(50 mg) was placed in a round-bottomed threeneckedflask and the system was purged with hydrogenfor 30 min. A solution of the substrate in ethanol 0.2 M(or in tetrahydrofuran in the case of using o- andp-dinitrobenzene as a substrate) was then poured intothe reactor by means of a feed cock of the reactionmixture. The concentration of platinum in the reactionmixture was 0.85 mol %. The reaction was carriedout with vigorous stirring on a magnetic stirrer at a rateof 900 rpm in the monitoring mode by means of gasliquidchromatography until the peak of the startingcompound disappeared on the chromatogram. At theend of the experiment, the stirring was stopped; thecatalyst was separated from the reaction mixture bycentrifugation.Analysis of the reaction products was performedusing GC with a Chromatek Crystal 5000.2 chromatographwith a flame ionization detector (FID) and acapillary column CR-5 (2 mm × 25 m) at a temperatureof 190°C. The peaks were identified on the basis of the experimentally obtained retention times of individualcompounds (nitrobenzene, o-dinitrobenzene,p-dinitrobenzene, p-hydroxynitrobenzene, p-nitroacetophenone,nitrocyclohexane, aniline, o-phenylenediamine,p-phenylenediamine, p-hydroxyaniline,p-nitrophenylethanol, aminocyclohexane). Thestructure and purity of the products obtained wereconfirmed by NMR spectroscopy. The 1H and 13CNMR spectra were recorded on a Bruker Avance 300spectrometer in solvents CDCl3 and DMSO-d6.100%With hydrazine hydrate In ethanol at 85℃ for 0.416667h99%With borane-ammonia complex In methanol; water at 20℃ for 0.0833333h99%With ammonia borane; Pd/MIL-101 In methanol; water at 20℃ for 0.05h99%
Safety and Hazards
Pictogram(s)




SignalDangerGHS Hazard StatementsH301: Toxic if swallowed
H311: Toxic in contact with skin
H317: May cause an allergic skin reaction
H319: Causes serious eye irritation
H331: Toxic if inhaled
H341: Suspected of causing genetic defects
H400: Very toxic to aquatic life
H410: Very toxic to aquatic life with long lasting effects
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P311, P312, P321, P322, P330, P333+P313, P337+P313, P361, P363, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationClass 6.1; Packaging Group: III; UN Number: 1673 Under the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD 7.5/kg
Use Pattern m-Phenylenediamine CAS 108-45-2 may be used in the synthesis of the following:
• intrinsically electrically semiconducting microparticles of semiladder poly(m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) structures
• extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite, used as sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls
• series of terpolymers, via chemical oxidative polymerization
• thin film composite (TFC) membranes based polyamide
• TFC reverse osmosis (RO) membranes m-Phenylenediamine (MPD) is an aromatic diamine. It copolymerizes with iso- or terephthaloyl chloride and 3,5-diaminobenzoic acid or 2,4-diaminobenzene sulfonic acid (or sulfonate) to afford copolyamides by low temperature solution polymerization. MPD has been reported to be formed selectively during the hydrogenation of m-dinitrobenzene in the presence of bimetallic Ni-Pt catalysts supported on carbon. Cyclic voltammetric method has been proposed to study the electrochemical copolymerization of aniline withMPD in sulfuric acid solution.
Read the full article
0 notes
Text
m-Phenylenediamine CAS#: 108-45-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Namem-PhenylenediamineIUPAC Namebenzene-1,3-diamineMolecular Structure
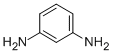
CAS Registry Number 108-45-2EINECS Number203-584-7MDL NumberMFCD00007799Beilstein Registry Number471357Synonymsm-phenylenediamine, 1,3-phenylenediamineMolecular FormulaC6H8N2Molecular Weight108.143InChIInChI=1S/C6H8N2/c7-5-2-1-3-6(8)4-5/h1-4H,7-8H2InChI KeyWZCQRUWWHSTZEM-UHFFFAOYSA-NCanonical SMILESC1=CC(=CC(=C1)N)N
Patent InformationPatent IDTitlePublication DateCN109134267A synthetic method of the compound aromatic amines (by machine translation) 2019CN109810019A compound and its preparation method and as a fluorescence sensor application (by machine translation) 2019CN108164452A double-(mild imide) preparation method (by machine translation) 2018CN109081793Sulfonamide urease inhibitor and its preparation and use (by machine translation)2018CN109096156Between the two sulfonyl aryl diamine urease inhibitor and its preparation and use (by machine translation)2018WO2017/130103PROCESS FOR PREPARING A TRANSITION METAL-SCHIFF BASE IMINE LIGAND COMPLEX2017CN104058993Photosensitive diamine, polyamide acid or the derivatives thereof, the liquid crystal aligning agent, liquid crystal orientation film and liquid crystal display element (by machine translation)2017
Physical Data
AppearanceColorless needle crystalVapor Density3.7 (vs air)Vapor Pressure0.62 mm Hg ( 100 °C)Flash Point>230 °FWater Solubility350 g/L (25 ºC)Pka5.11, 2.50(at 20℃)
Melting Point, °C Solvent (Melting Point) 65toluene, cyclohexane64 - 67
Boiling Point, °CPressure (Boiling Point), Torr1401014622282 - 284760
Sublimation, °CPressure (Sublimation), Torr100 - 1104
Density, g·cm-3Reference Temperature, °C Measurement Temperature, °C1.05541251.07741001.0844901.138915151.13372525
Refractive IndexWavelength (Refractive Index), nmTemperature (Refractive Index), °C1.62558656.357.71.633958957.71.6761743457.7
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d125600Chemical shifts, Spectrum13Cchloroform-d125150Chemical shifts1Hchloroform-d124.84300Chemical shifts, Spectrum13Cchloroform-d124.8475
m-Phenylenediamine CAS#: 108-45-2 NMR
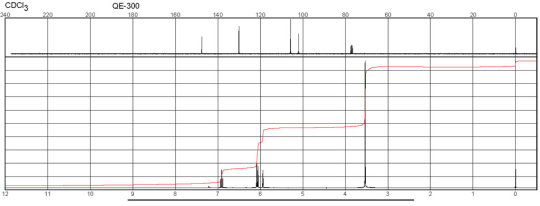
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Intensity of IR bands, Bands, Spectrumsodium chlorideBandspotassium bromidem-Phenylenediamine CAS#: 108-45-2 IR

m-Phenylenediamine CAS#: 108-45-2 Raman
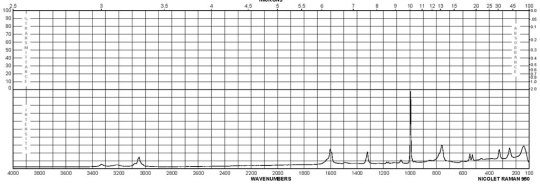
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1acetonitrile215, 29558100, 4900Spectrumethanol200 - 250 nmAbsorption maximaethanol2922300Spectrumethanol250 - 450 nmAbsorption maximaH2O289, 238, 2101995, 7244, 32359Absorption maximamethanol 292.5, 242.5, 213.52291, 7079, 30199
Route of Synthesis (ROS)

Route of Synthesis (ROS) of m-Phenylenediamine CAS 108-45-2
ConditionsYieldWith sodium tetrahydroborate In water at 20℃ for 1h100%With hydrogen; sodium fluoride In methanol at 39.84℃ for 2.17100%With hydrogen In ethanol at 20℃ under 760.051 Torr for 3h chemoselective reaction
Experimental Procedure
General procedure: In this work, the following of nitroaromatic compoundswere used:The liquid-phase hydrogenation reaction of nitroaromaticcompounds can be presented as:It was carried out at room temperature under an atmosphericpressure of H2. A sample of the catalyst(50 mg) was placed in a round-bottomed threeneckedflask and the system was purged with hydrogenfor 30 min. A solution of the substrate in ethanol 0.2 M(or in tetrahydrofuran in the case of using o- andp-dinitrobenzene as a substrate) was then poured intothe reactor by means of a feed cock of the reactionmixture. The concentration of platinum in the reactionmixture was 0.85 mol %. The reaction was carriedout with vigorous stirring on a magnetic stirrer at a rateof 900 rpm in the monitoring mode by means of gasliquidchromatography until the peak of the startingcompound disappeared on the chromatogram. At theend of the experiment, the stirring was stopped; thecatalyst was separated from the reaction mixture bycentrifugation.Analysis of the reaction products was performedusing GC with a Chromatek Crystal 5000.2 chromatographwith a flame ionization detector (FID) and acapillary column CR-5 (2 mm × 25 m) at a temperatureof 190°C. The peaks were identified on the basis of the experimentally obtained retention times of individualcompounds (nitrobenzene, o-dinitrobenzene,p-dinitrobenzene, p-hydroxynitrobenzene, p-nitroacetophenone,nitrocyclohexane, aniline, o-phenylenediamine,p-phenylenediamine, p-hydroxyaniline,p-nitrophenylethanol, aminocyclohexane). Thestructure and purity of the products obtained wereconfirmed by NMR spectroscopy. The 1H and 13CNMR spectra were recorded on a Bruker Avance 300spectrometer in solvents CDCl3 and DMSO-d6.100%With hydrazine hydrate In ethanol at 85℃ for 0.416667h99%With borane-ammonia complex In methanol; water at 20℃ for 0.0833333h99%With ammonia borane; Pd/MIL-101 In methanol; water at 20℃ for 0.05h99%
Safety and Hazards
Pictogram(s)




SignalDangerGHS Hazard StatementsH301: Toxic if swallowed
H311: Toxic in contact with skin
H317: May cause an allergic skin reaction
H319: Causes serious eye irritation
H331: Toxic if inhaled
H341: Suspected of causing genetic defects
H400: Very toxic to aquatic life
H410: Very toxic to aquatic life with long lasting effects
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P311, P312, P321, P322, P330, P333+P313, P337+P313, P361, P363, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationClass 6.1; Packaging Group: III; UN Number: 1673 Under the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD 7.5/kg
Use Pattern m-Phenylenediamine may be used in the synthesis of the following:
• intrinsically electrically semiconducting microparticles of semiladder poly(m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) structures
• extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite, used as sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls
• series of terpolymers, via chemical oxidative polymerization
• thin film composite (TFC) membranes based polyamide
• TFC reverse osmosis (RO) membranes m-Phenylenediamine (MPD) is an aromatic diamine. It copolymerizes with iso- or terephthaloyl chloride and 3,5-diaminobenzoic acid or 2,4-diaminobenzene sulfonic acid (or sulfonate) to afford copolyamides by low temperature solution polymerization. MPD has been reported to be formed selectively during the hydrogenation of m-dinitrobenzene in the presence of bimetallic Ni-Pt catalysts supported on carbon. Cyclic voltammetric method has been proposed to study the electrochemical copolymerization of aniline withMPD in sulfuric acid solution.
Read the full article
0 notes
Text
m-Phenylenediamine CAS#: 108-45-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Namem-PhenylenediamineIUPAC Namebenzene-1,3-diamineMolecular Structure
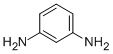
CAS Registry Number 108-45-2EINECS Number203-584-7MDL NumberMFCD00007799Beilstein Registry Number471357Synonymsm-phenylenediamine, 1,3-phenylenediamineMolecular FormulaC6H8N2Molecular Weight108.143InChIInChI=1S/C6H8N2/c7-5-2-1-3-6(8)4-5/h1-4H,7-8H2InChI KeyWZCQRUWWHSTZEM-UHFFFAOYSA-NCanonical SMILESC1=CC(=CC(=C1)N)N
Patent InformationPatent IDTitlePublication DateCN109134267A synthetic method of the compound aromatic amines (by machine translation) 2019CN109810019A compound and its preparation method and as a fluorescence sensor application (by machine translation) 2019CN108164452A double-(mild imide) preparation method (by machine translation) 2018CN109081793Sulfonamide urease inhibitor and its preparation and use (by machine translation)2018CN109096156Between the two sulfonyl aryl diamine urease inhibitor and its preparation and use (by machine translation)2018WO2017/130103PROCESS FOR PREPARING A TRANSITION METAL-SCHIFF BASE IMINE LIGAND COMPLEX2017CN104058993Photosensitive diamine, polyamide acid or the derivatives thereof, the liquid crystal aligning agent, liquid crystal orientation film and liquid crystal display element (by machine translation)2017
Physical Data
AppearanceColorless needle crystalVapor Density3.7 (vs air)Vapor Pressure0.62 mm Hg ( 100 °C)Flash Point>230 °FWater Solubility350 g/L (25 ºC)Pka5.11, 2.50(at 20℃)
Melting Point, °C Solvent (Melting Point) 65toluene, cyclohexane64 - 67
Boiling Point, °CPressure (Boiling Point), Torr1401014622282 - 284760
Sublimation, °CPressure (Sublimation), Torr100 - 1104
Density, g·cm-3Reference Temperature, °C Measurement Temperature, °C1.05541251.07741001.0844901.138915151.13372525
Refractive IndexWavelength (Refractive Index), nmTemperature (Refractive Index), °C1.62558656.357.71.633958957.71.6761743457.7
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d125600Chemical shifts, Spectrum13Cchloroform-d125150Chemical shifts1Hchloroform-d124.84300Chemical shifts, Spectrum13Cchloroform-d124.8475
m-Phenylenediamine CAS#: 108-45-2 NMR
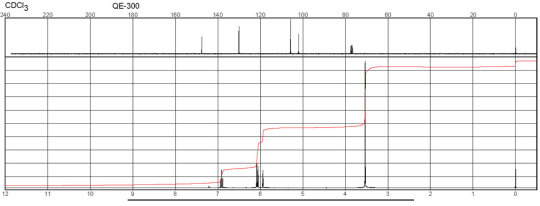
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Intensity of IR bands, Bands, Spectrumsodium chlorideBandspotassium bromidem-Phenylenediamine CAS#: 108-45-2 IR
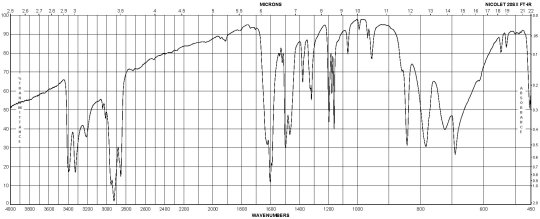
m-Phenylenediamine CAS#: 108-45-2 Raman
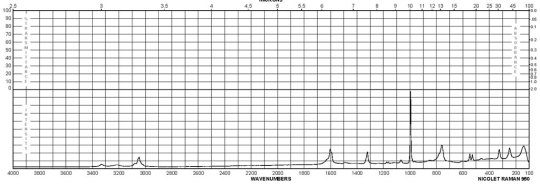
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1acetonitrile215, 29558100, 4900Spectrumethanol200 - 250 nmAbsorption maximaethanol2922300Spectrumethanol250 - 450 nmAbsorption maximaH2O289, 238, 2101995, 7244, 32359Absorption maximamethanol 292.5, 242.5, 213.52291, 7079, 30199
Route of Synthesis (ROS)

Route of Synthesis (ROS) of m-Phenylenediamine CAS 108-45-2
ConditionsYieldWith sodium tetrahydroborate In water at 20℃ for 1h100%With hydrogen; sodium fluoride In methanol at 39.84℃ for 2.17100%With hydrogen In ethanol at 20℃ under 760.051 Torr for 3h chemoselective reaction
Experimental Procedure
General procedure: In this work, the following of nitroaromatic compoundswere used:The liquid-phase hydrogenation reaction of nitroaromaticcompounds can be presented as:It was carried out at room temperature under an atmosphericpressure of H2. A sample of the catalyst(50 mg) was placed in a round-bottomed threeneckedflask and the system was purged with hydrogenfor 30 min. A solution of the substrate in ethanol 0.2 M(or in tetrahydrofuran in the case of using o- andp-dinitrobenzene as a substrate) was then poured intothe reactor by means of a feed cock of the reactionmixture. The concentration of platinum in the reactionmixture was 0.85 mol %. The reaction was carriedout with vigorous stirring on a magnetic stirrer at a rateof 900 rpm in the monitoring mode by means of gasliquidchromatography until the peak of the startingcompound disappeared on the chromatogram. At theend of the experiment, the stirring was stopped; thecatalyst was separated from the reaction mixture bycentrifugation.Analysis of the reaction products was performedusing GC with a Chromatek Crystal 5000.2 chromatographwith a flame ionization detector (FID) and acapillary column CR-5 (2 mm × 25 m) at a temperatureof 190°C. The peaks were identified on the basis of the experimentally obtained retention times of individualcompounds (nitrobenzene, o-dinitrobenzene,p-dinitrobenzene, p-hydroxynitrobenzene, p-nitroacetophenone,nitrocyclohexane, aniline, o-phenylenediamine,p-phenylenediamine, p-hydroxyaniline,p-nitrophenylethanol, aminocyclohexane). Thestructure and purity of the products obtained wereconfirmed by NMR spectroscopy. The 1H and 13CNMR spectra were recorded on a Bruker Avance 300spectrometer in solvents CDCl3 and DMSO-d6.100%With hydrazine hydrate In ethanol at 85℃ for 0.416667h99%With borane-ammonia complex In methanol; water at 20℃ for 0.0833333h99%With ammonia borane; Pd/MIL-101 In methanol; water at 20℃ for 0.05h99%
More: Inquiry all available synthetic routes with detailed experimental procedures
Safety and Hazards
Pictogram(s)




SignalDangerGHS Hazard StatementsH301: Toxic if swallowed
H311: Toxic in contact with skin
H317: May cause an allergic skin reaction
H319: Causes serious eye irritation
H331: Toxic if inhaled
H341: Suspected of causing genetic defects
H400: Very toxic to aquatic life
H410: Very toxic to aquatic life with long lasting effects
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P311, P312, P321, P322, P330, P333+P313, P337+P313, P361, P363, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationClass 6.1; Packaging Group: III; UN Number: 1673 Under the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life2 yearsMarket PriceUSD 7.5/kg
Use Pattern m-Phenylenediamine may be used in the synthesis of the following:
• intrinsically electrically semiconducting microparticles of semiladder poly(m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) structures
• extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite, used as sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls
• series of terpolymers, via chemical oxidative polymerization
• thin film composite (TFC) membranes based polyamide
• TFC reverse osmosis (RO) membranes m-Phenylenediamine (MPD) is an aromatic diamine. It copolymerizes with iso- or terephthaloyl chloride and 3,5-diaminobenzoic acid or 2,4-diaminobenzene sulfonic acid (or sulfonate) to afford copolyamides by low temperature solution polymerization. MPD has been reported to be formed selectively during the hydrogenation of m-dinitrobenzene in the presence of bimetallic Ni-Pt catalysts supported on carbon. Cyclic voltammetric method has been proposed to study the electrochemical copolymerization of aniline withMPD in sulfuric acid solution.
Read the full article
0 notes
