#hydroboration
Photo
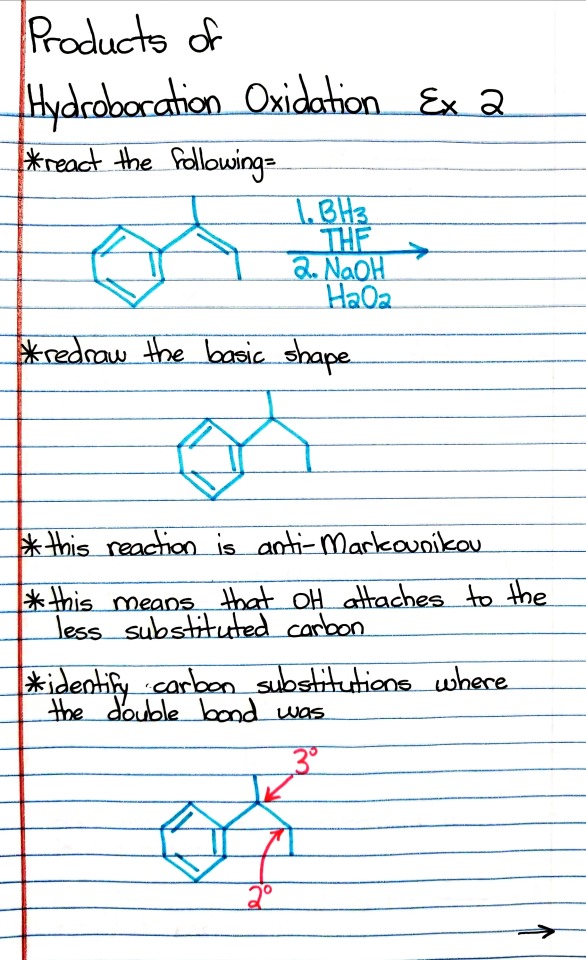


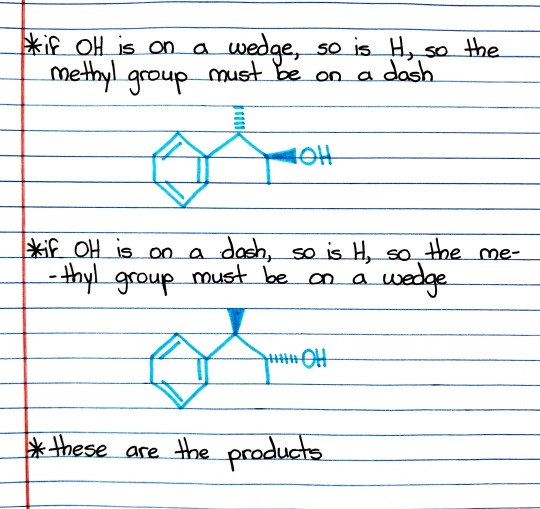
#organic chemistry#ochem#o-chem#o chem#ochem 1#ochem 2#organic chemistry 1#organic chemistry 2#mcat organic chemistry#orgo#reactions#orgo reactions#chemical reactions#orgo mcat#mcat orgo#ochem reactions#studyblr#notes#hydroboration oxidation#hydroboration-oxidation
4 notes
·
View notes
Text

1 note
·
View note
Text
guys i miss organic chemistry guys that was the best class ever guys hey hey are you listening to me do you wanna hear about hydroboration can i tell you about the implications of enantiomers in medicine hey wait come back
14 notes
·
View notes
Text
okay so the wifi i'm on right now is shitting itself despite this being a popular study spot on campus but REGARDLESS i'm studying for my third organic chemistry midterm right now (my class grade is a 90.2% so i'm barely hovering around an A and i'd REALLY like to keep it that way as unrealistic as that may be) and as much as i am absolutely loving this class i'm struggling a little with remembering all of the mechanisms (final exam review sheet posted by professor indicates 40 different reactions we need to know).
granted, a lot of them share the same patterns, but i don't know what format i'll need to compare-contrast all of them in in order to have them down pat for may 2. so right now i'm just focusing on this exam, where we've gotta know
radical substitution of alkanes and allylic substitution, the funky alkyl halide ones with the fishhook arrows. the simplified mechanism looks like halogenation except these are done with heat/light. these go through three stages: initiation from nonradical to radical, propagation from radical to radical, and termination from radical to nonradical. you get your radical intermediate via homolytic cleavage, you react it with a hydrogen coming off of the most substituted carbon, you get your next radical intermediate once the hydrogen is gone, you react all your radicals together to tie things up nicely. if your radical is on a carbon that neighbors a double bond, you have a nice stable allylic radical (thank you resonance), but if your radical is on a double bond, that's a vinyl radical and we don't like those... you also gotta pay attention to where your alkene is because that'll affect your stability too. if you're happening to do your reaction with peroxides, you'll be reacting your intermediate with the less substituted area. a halogen? on MY terminal end?
elimination and substitution reactions Sn1/Sn2/E1/E2. these are silly and complicated and i need to make a chart to understand them better, but the substitution reactions will substitute a nucleophile in for the leaving group, and the elimination reactions will make an alkene out of an alkane using a base, and the 2-reactions are concerted with steric barriers while the 1- reactions have a carbocation intermediate with stability as the barrier.
alkyne reactions, including deprotonation (basically acid-base shit), alkyne formation (using acetylene, which makes good internal alkenes and looks like Sn2, or dibromides, which looks like two E2 reactions and can be done with bromides on the same carbon or on adjacent carbons), halogenation (nonregioselective concerted anti-addition of halogens using Br2/Cl2), hydrohalogenation (markovnikov addition of Br, can happen twice if you have two equivalents of HBr, carbocation intermediate), hydration and hydroboration-oxidation (markov and anti-markov reactions, respectively; adding a hydrogen and an OH, keto-enol tautomerism where you end up with the double bond on the oxygen), and three reduction reactions (Pd/C yields alkane, Lindlar/H2 yields a cis(z)alkene, Na/NH3 yields a trans(E) alkene).
anyway i have practice problems to do and the sn1/sn2/e1/e2 reaction comparison chart to make but i'm drinking my first monster energy (ultra paradise. it's good) and i'm getting dinner w at least one of my friends in 25 minutes... calc homework still due tonight but i think i'll be able to get it... exam in 3 hours... feeling good!
#a rare brain dump from puzzlehat but making notecards was not being an efficient way to study lawl#perhaps i should make a studyblr...
5 notes
·
View notes
Text
heat of hydrogenation mesomeric effect markovnikoff's rule dancing resonance oxymercuration demercuration dehydrohalogenation birch reduction rate of decarboxylation hydroboration prileschieav reaction




5 notes
·
View notes
Note
(trying to get my name into your tags by playing send words) hydroboration
what does that even MEAN
2 notes
·
View notes
Text
Borane Dimethyl Sulphide Complex: Unleashing Versatility in Organic Synthesis by Symax Labs
Symax Labs, a distinguished name in the realm of chemical innovation, proudly introduces its Borane Dimethyl Sulphide (BMS) Complex. This sophisticated reagent has garnered attention for its versatility and efficiency in a wide array of organic synthesis applications. In this article, we delve into the unique features and applications of Symax Labs' Borane Dimethyl Sulphide Complex.

Key Features of Borane Dimethyl Sulphide Complex:
High Reactivity: Symax Labs' BMS Complex is renowned for its exceptional reactivity, making it a powerful reducing agent and a versatile tool in various synthetic transformations.
Mild Reaction Conditions: This complex operates under mild reaction conditions, ensuring the preservation of sensitive functional groups and enhancing its compatibility with a diverse range of substrates.
Selectivity: The Borane Dimethyl Sulphide Complex exhibits high chemoselectivity and regioselectivity, providing precise control over the course of reactions and facilitating the synthesis of complex molecules.
Compatibility with Aqueous Conditions: Unlike some boron-based reagents, Symax Labs' BMS Complex exhibits compatibility with aqueous conditions, expanding its utility in aqueous-phase reactions.
Applications in Organic Synthesis:
Reduction of Carbonyl Compounds: The Borane Dimethyl Sulphide Complex is a powerful reducing agent for the selective reduction of various carbonyl compounds, including ketones, aldehydes, and carboxylic acids.
Hydroboration Reactions: It finds application in hydroboration reactions, allowing chemists to selectively introduce boron to unsaturated compounds with excellent regio- and stereoselectivity.
Metal-Free Reductions: Symax Labs' BMS Complex offers an alternative to metal-based reducing agents, making it an attractive choice for metal-sensitive reactions.
Synthesis of Organoboranes: The reagent is instrumental in the synthesis of organoboranes, which serve as valuable intermediates in the preparation of various functionalized compounds.
Symax Labs: Elevating Organic Synthesis to New Heights
Symax Labs' commitment to innovation is exemplified by its Borane Dimethyl Sulphide Complex. This reagent empowers researchers and synthetic chemists with a reliable and efficient tool to address complex synthetic challenges.
Conclusion:
As the demand for versatile and high-performance reagents continues to grow, Symax Labs remains at the forefront of delivering innovative solutions to the scientific community. The Borane Dimethyl Sulphide Complex stands as a testament to Symax Labs' dedication to advancing organic synthesis, offering a reliable and powerful tool that opens new avenues for groundbreaking discoveries in the field of chemistry. Researchers can confidently rely on Symax Labs for precision, quality, and excellence in chemical synthesis.
0 notes
Text
Alkene to alcohol
Hydroboration oxidation - anti markonikov addition
Oxymercuration demurcuration - markonikov without rearrangement
0 notes
Video
youtube
Hydroboration Oxidation Alkene reaction mechanism
0 notes
Text
One more note before bed bc now I'm excited about Boron.

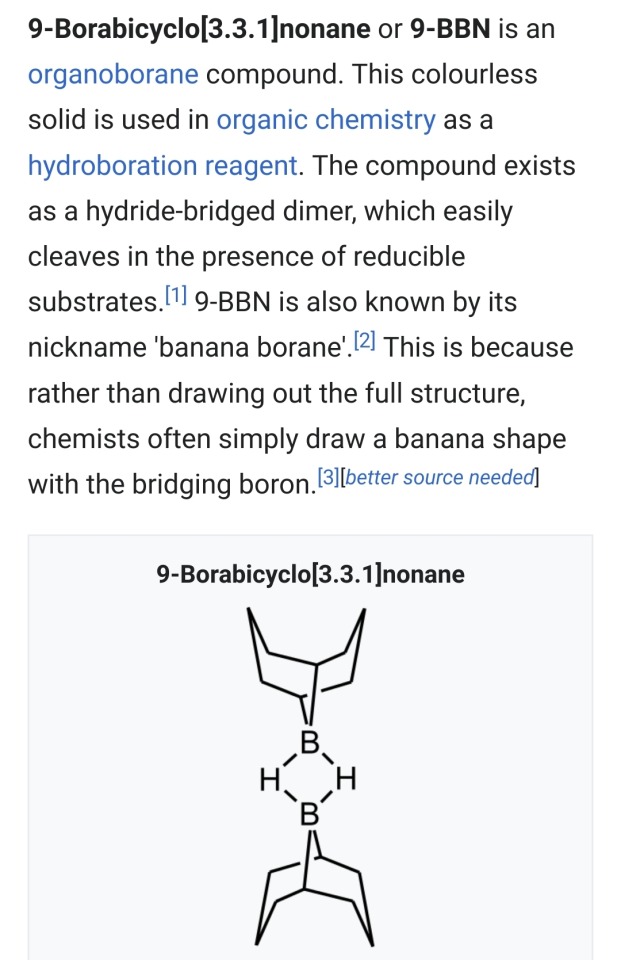
Its in my top fave elements (B, F, V, Xe) because its breaks ALL the rules by being the only nonmetal with an empty suborbital. B and Si (as I mentioned in the last post) are "secret elements", theyre not the CHONSPX you'll find in organic products, but they're stable enough and indispensable in the lab. None of this is important right now, though, because we're making fun of
Banana Borane
With a side of
Bis(Piña Colada) diboron!
Thats the whole post, they're useful for *long list of Boron-based chemistry* I just thought the names were fucking hilarious. Unfortunately bis(pinacolato)diboron gets its name from pinacol, 2,3-dimethylbutane-2,3-diol, not from the fact that each half looks like a glass you'd drink a Piña Colada out of.
In the immortal words of Wakko Warner, "Gooooodnight everybody!"
#biochem#biochemistry#chemistry#studyblr#learning#nerd#organic chemistry#science#chem#boron#pina colada#piñacolada#banana#borane#hydroboration
1 note
·
View note
Text
Hi friends! I’m kicking myself offline for the next two weeks to focus on finishing my summer classes strong. See y’all soon, and wish me luck!
#class is over on the 7th so expect me around then#see y'all soon xoxo#if an asides comes out i'll be back but other than that :p#message in a bottle#if you see me online yell at me to go study the hydroboration oxidation mechanism#plz orgo is very important for med school
61 notes
·
View notes
Photo




My students purify one of their reactions using a Kugelrohr short path distillation and the purified product has the most amazing crystals!
113 notes
·
View notes
Text
dont underestimate my ochem insanity i snuck a hydroboration reaction into my fanart once just cuz i wanted to
8 notes
·
View notes
Note
2 with beau? ❤️

Happy almost end of blurb weekend!
Tagging: @besthockeyfics @glassdanse @calgarycanuck @stlbluesbrat21 @dembenchboys @nhlboyshavemyhart88 @stars-canucks @gotpucks @beauvibaby
2.“I won’t let you do this alone.”
Warning: contains science
Word Count: 557
____________________________
You let out a groan so loud it was practically a scream. Nothing was more frustrating to you than studying for an exam, something that you told yourself you would never do again once you graduated college. Yet, there you were, sitting at the kitchen table, flashcards, blank pieces of computer paper, three open textbooks, and upwards of 100 colored pens scattered around you and your computer, studying for an exam.
“I like those groans better when they come from the bedroom,” Anthony says, poking his head into the kitchen. “They’re more fun when you make them because you’re happy.”
He comes up behind you, rubbing your shoulders in an attempt to relax you. “Why the fuck did I think it was a good idea to get a master’s degree?”
“Because you'll have more knowledge and make more money.”
“Ok, but why the fuck did I think it was a good idea to get one in chemistry of all subjects? Who likes chemistry?”
“You do. You have piles of notes next to our bed and you read them for fun before we go to sleep each night.”
“Fuck, I do, don’t I,” you groan, putting your head down on the table. “This is the worst idea I’ve ever had.”
“Hey, you’re going to be fine,” your boyfriend says, sitting down next to you and grabbing a stack of your flashcards. “I’m sure you know more than you think. I’ll quiz you.”
“Are you even going to know if I’m right?”
“If you made the flashcards right, then I’m sure I can figure it out,” he says, tapping them on the table to get them all even, flipping through the first few of them. “I’m not going to let you do this alone.”
“Fine,” you say begrudgingly, not sure this was even going to work.
“Alright, what are the two ways to prepare an alcohol from 1-methyl-1-cyclohexene?” he starts, struggling through the name of the compound. “This isn’t English.”
“You’re right, it’s organic chemistry. It’s a whole other language.”
“Well, what are they, though?”
You groan, knowing that he was going to have no idea what you were about to tell him. “The first is hydroboration, which is oxidation, and it’s syn-addition, and follows an anti-Markovnikov hydration. So the BH3 attacks the cyclohexene, and one hydrogen adds on to the same carbon that has the methyl group attached to it, while the BH2 attacks to the other carbon involved in the double bond, trans to the methyl, forming the intermediate. Then hydrogen peroxide attacks and the hydroxide replaces the boron group and yields trans-2-methylcyclohexanol,” you rant, Anthony nodding along as you explain to him the second one.
“Yes, fuck, babe, you got it perfectly!” Anthony yells, getting up to kiss you. “How about, every question you get right, you get a reward from me?”
“I like incentives, but it depends on what they are,” you tell him, giving him another kiss before he sits back down.
“Every question you get right, is one night where you have complete control in the bedroom. Anything you want, I will do, no questions asked,” he offers, taking one of the unused sheets of printer paper to keep tally.
“I like the sound of that,” you tell him. “What if I get it wrong?”
“I have control.”
“Deal. Ask me another question.”
#tito beauvillier#tito beauvillier imagines#anthony beauvillier#anthony beauvillier imagines#blurb weekend 521#new york islanders#new york islanders imagines
63 notes
·
View notes
Text
Hydroboration-oxidation: long story short
This is one of the big reactions; professors love it and asking questions about it. You’ll likely need to know the mechanism for an undergraduate test. Thankfully, I’m not really expected to draw that out on the GRE, though it’ll be good to be familiar with it. So here are some fast facts:
Overall transformation: Alkene into an alcohol; alkyne into an enol into an aldehyde via tautomerization.
type of reaction: a two-step electrophilic additon. technically a hydration--net gain of water. IT IS NOT A REDUCTION OR AN OXIDATION (the “oxidation” part of the name comes from an internal oxidation of the hydroborated step).
It is anti-Markovnikov and you’ll likely need to know that.
RETENTION OF STRUCTURE!!!!!!!!!
syn addition--the OH and H will add to the same side
reagents: To an alkene or alkyne, add in the first step BH3/THF. second step, add base and peroxide.
interesting notes about mechanism: the big deal here is that the hydrogens on BH3 all get displaced by the alkene (specifically on the less sterics side-->that’s why it’s antimarkovnikov). the base in the second step then comes in to steal that R group. (look at the mechanism will be more helpful tbh)
5 notes
·
View notes