#nerd
Text
Nerdy subs are something I will never shut up about.
Listening to them talk and talk and talk while I smile and think about how I'm going to make their brains shut down in a few minutes. Thinking about their pretty voices begging me for more, thinking about their trembling whimpers, thinking about how cute they would look if I put them in their place. I just want to grab a cute nerdy guy by the hair and force him to his knees, pull his collar and make him follow me like the smart boy he is. I want them to infodump me while my hand slowly goes up their thighs and then see their confused face when they realize what I did.
Oh, and by the way, if you cupped a nerdy guy's face with your hands and pulled him closer, teased him by almost kissing him but never actually doing it, and then grinned while looking into their eyes, they will shut off their brain and become stupid for you.
#bd/sm mommy#sub men#boy sub#male sub#men sub#puppy sub#sub puppy#subboy#subboys#subby boys#subby men#dom mommy#mommy k!nk#bd/sm mistress#domme mommy#bd/sm blog#bd/sm puppy#bd/sm kink#bd/sm community#bdsmplay#bdsmkink#bdsmblog#pet pl4y#pet pl@y#bd/sm pet#nerd#fdom#soft fem dom#femdxm#gentle fdom
457 notes
·
View notes
Text

#cute egirl#egirl aesthetic#sexy egirl#hot egirls#egirl#egirl noods#nerdy girls#hot nerd#sexy nerd#nerd#geeky girls#sexy geek#hot geek#geek girl#curvy blonde#curvy and beautiful#curvy and cute#chubby#curvy chicks#curvybeauty#thick and juicy#blond girl#curvy#curvy af#thickaf
181 notes
·
View notes
Text

Underwear
check here if ur also... what did the bros say again?: https://boog-how.carrd.co/
#booghowart#not an ask#booghow short comic#short comic#walter and vince#wav#vince#walter#gay#lgbt#comic#werewolf#vampire#furry art#jock#nerd#alright everyone's here hawld awn#willy#winston#wesley#boone#wallace#mandy#moody#demonica#coach max#detention
97 notes
·
View notes
Text
#draculaura#sanji x reader#agere community#joy division#meow#risa yukihira#ruby rose#nerd#beats#baldur's gate 3#buffy#Jason Grace#archery
124 notes
·
View notes
Text
#bookworm#cardcaptor sakura#tower of god#rick sanchez#draculaura#agere community#joy division#risa yukihira#x reader#nerd#padfoot#davekat#konosuba#chat blanc
131 notes
·
View notes
Text
#mlk#risa yukihira#nerd#eclectic witch#chat blanc#turtle#gouache#felice fawn#alec lightwood#brainwashing#ymcmb#loona
120 notes
·
View notes
Text

I worked a playoff Ontario Reign game where they won 5 to 1 and the LA Kings won in OT with Kopitar scoring the game winning goal so I’m a happy hockey fan today 🖤🏒
#halloween is a lifestyle#halloween is cool#me#selfie#hazel eyes#emo#nerd#love#smile#halloween#hockey#hockey fan#I love hockey#los angeles kings#La kings#Ontario reign#nhl#ahl#hockey jersey#anze kopitar#Calder cup playoffs#stanley cup playoffs
45 notes
·
View notes
Text

#marvel#nerd#ucm#mcu#pop#iron man#homem de ferro#tony stark#stark#robert downey jr#anthony edward stark
34 notes
·
View notes
Text
Lion El'Jonson has donned the "frost" armor again!
Do You like this original painting?
If you want to get this or similar one for yourself -> link in bio.
#eljonson#miniaturepainting#PaintingWarhammer#warhammer40k#paintings#warhammer#TabletopGaming#modelpainting#darkangels#geek#terminators#primaris#lion#best4minis#primarch#tabletopminiatures#nerds#wargaming#WarhammerCommunity#gamesworkshop#wh40k#fallen#SpaceMarines#ImperiumOfMan#WarhammerPainting#minipainting#warhammer40000#nerd#coolminis#warmongers
33 notes
·
View notes
Text




Hmmm idk about this filter 🤔😬🤨
#alt girl#goth girl#alternate universe#alternative#gothic#goth aesthetic#makeup#makeup looks#goth makeup#goth#dc comics#dc universe#thick goth#thick babe#thickwomen#filter#me#curvy girls#nerd#nerdy girls
39 notes
·
View notes
Text
YOOO NEW CARDS

#hazbin alastor#hazbin hotel#hazbin lucifer#radioapple#hazbin angel dust#hazbin charlie#hazbin hotel lucifer#credit card debt#nerd#the radio demon#ace in the hole
37 notes
·
View notes
Text
"Why are there so many female archers in fiction?"
Please forgive the clickbait-y title! This is a super complex and interesting topic that I barely scratch the surface of here, but I hopefully will be able to do more justice to things like this in the future!
Also, it's not the point of the video, but I had fun with the outfits in this- do you have any faves?
As always, please consider supporting me on Patreon if you can, or watching on youtube if not!
68K notes
·
View notes
Text
3K notes
·
View notes
Text
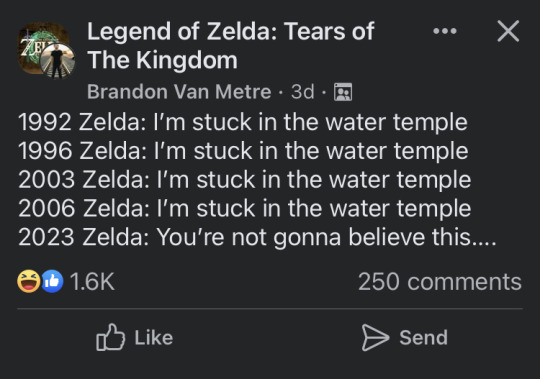
13K notes
·
View notes
Text

Oh hey, it is those famous slimes :)
@kurbiismind
#art#artwork#digital art#artists on tumblr#drawing#suggestive#slime girl#monster girl#get attacked#nerd
3K notes
·
View notes
