Text

FRIENDLY REMINDER TO ANY NEW FOLLOWERS, PLEASE FOLLOW MY NEW TUMBLR! (tumblr.com/mycochaotix).
I do not update this tumblr any-longer and all new content (like some I am working on releasing in the next few hours) will only post on the new tumblr!
@mycochaotix
Thanks mycopals!
2 notes
·
View notes
Text

Hey there guys, gals, and non-binary mycopals!
Mycothaotix here, and this shall be my first post on my new, separated tumblr-blog. I was not able to respond to reblog questions and didnt have a way to impliment 'ask's on my previous blog (now titled: 'mycochaos-oldacnt-plzfollownew).
I have some visible old posts under the #mycochaotix tag that I usually use for all posts (and plan to continue to use).
I consider myself a Non-Binary, He/they mycophile and have 577 days of experience (as of 7/24/23) in mycological research and cultivation. I also serve as a moderator on the Contamination ID and Myco-advice subreddit r/ContamFam. On that platform, I am known as the Trich Hunter. I have a passion for studying all types of contamination (in addition to primarily researching basidiomycete fungi) because I believe that learning from failures and understanding what to avoid in research efforts are crucial for personal growth and reducing occurrences of contamination.
Please follow for more!
Message me on IG or Reddit with any questions; or to share your mycology successes and failures! 🍄 u/Dry_Cardiologist8370 IG: myco_chaotix Tumblr: myocochaotix
12 notes
·
View notes
Text

#tumblr memes#mycology#myco#gay#mushroom#art#mycelium#fungi#mushies#mycochaos#nature#magic mushies#pscilocin#pscilocybin#paul stamets#beauty#pgt#cute#albino penis envy#ape#lgbt
3 notes
·
View notes
Text








Making a GIF later today after I get final flush pic later today after work :) this is about a week of pinning then maturity :)
IG: myco_chaotix
u/Dry_Cardiologist8370
#mycology#myco#gay#mushroom#art#fungi#mycelium#mushies#mycochaos#magic mushies#natalensis#p nat#psilocybe#psilocybe natalensis#substrate#spore#nature#time lapse stills#lgbt#enby#myco chaotix#myocchaotix#myco chaos
12 notes
·
View notes
Text



Some of my AI art from the last year :) If you arent following me on Instagram, you are missing the most active part of my social media content push at the moment! I try to post daily tub updates of my research efforts and other useful pictographic explainers and sometimes AI art :) IG: myco_chaotix
#mcx#mycology#nature#ai#ai art#beauty#beautiful#mycelium#mushroom#mushies#gay#lgbt#chaos#space#the void
4 notes
·
View notes
Text
July Mycopal Q&A
Q: “ Hi do you mist the cake or just its side walls to fruit...i exsperment with a all in one grow bag..and another i have a water bin were the cake sits on brick abouve water...but do i ned to ever spray the cake its self ..or just side walls n lid.“
A:
hello mycopal! sorry for the late reply! I don't seem to get notifications for comments on my own videos :( I apologize for the length of this message, but I'm passionate about this topic, and I appreciate your genuine question! After reading it, fel free to let me know if you have any follow-up questions or thoughts. I'm also open to suggestions for content you would watch if I created said content related to mycology (with some cross over into microbiology).
I suggest, if you haven't already, get yourself a hair-stylist's misting spray bottle for your mycojourney :) I recommend using a misting bottle because pressurized spraying, even with a fine nozzle salon misting bottle, can dislodge spores of contaminant fungi from their microscopic structures. Additionally, water splashing can spread bacteria, which usually requires transfer from the user's skin or falling off the nose, eyebrows, hands, etc., or through water transfer. Some bacteria have flagella and can wiggle around, but not very far on dry surfaces.
I strongly advise against directly spraying the surface of the mycelial colony or any mycelium. Instead, only mist the sides and underside of the lid when they become dry. This should not happen often if you maintain proper field capacity. In general, direct spraying (and sometimes even just air flow) can cause mycelium to bruise.
Bruising isn't necessarily "bad," but if an area of the colony is severely bruised for an extended period, it can hinder the mycelium's metabolism in that specific area and even stall the growth of the entire tub. When you're breaking up grainspawn and mixing it with substrate to "birth" it into a tub (often referred to as S2B), it's important to understand the concept of "field capacity" (FC) in substrates.
If you're confident about your FC levels and have control over your spawn, substrate, and other variables, the substrate and colonized spawn/sub mix should maintain humidity within the tub itself without the need to remove any lids or introduce misting. This advice specifically applies if you're using shoeboxes or other plastic tote-tubs, and it requires some adjustments to the methods used when working with an AIO bag.
In the case of an all-in-one (AIO) bag, which will have reduced flush sizes/pin spread by the nature of available surface to do so), you have no way to confirm its sterility or exercise quality control (unless you made the bag yourself). You CAN control contamination at the injection site and in your local environment where you inoculate/inject the AIO bag through its injection port. However, you do not control the sterility of the syringe fluid you are injecting or the cleanliness and correctness of spores or mycelial tissue if using a liquid culture syringe. If you have the opportunity, using LC syringes is much better ( I have more reasons but will refrain cause this comment is already too wordy). Multi spore syringes I consider, very much, chaotic "luck of the draw". The single spores must meet a pair-mate, and their coupling results in the first initial mycelium hyphae that then spreads and colonizes.
So, technically, the mycelium 'colony' is an individual organism and the mushrooms are just the final stage of the life cycle of the fungal organism :)
BUT -- Do not be dismayed: AIO bags do work; but I do not use them due to the reasons mentioned above. Worth noting that grain-bags can be useful for those with limited space or time/resource constraints, and some gourmet mushroom species require fruiting from blocks of colonized spawn (not so much with AIO bags). On r/contamfam (where I have the privilege and pleasure of being a member of the mod-team), we have mycopals who have entered our summer full canopy contest with impressive canopies grown on small 4x2 blocks pulled from an AIO bag or similar sizes.
However, I strongly encourage all mycopals I advise to work towards doing as much of the hobby themselves as possible. There is a lot of variability in substrates, and you can often find materials at your local farmer's cooperative or grain/feed stores. Additionally, if you're interested in exploring woodlovers, you can often source woodchips from local mills or other available sources.
As I have mentioned before, if you can afford it, I highly recommend transitioning to using shoeboxes. They are 6qt Sterilite tubs designed for long-term sneaker storage. They have a passive FAE (fresh air exchange) at the lid-clasp/handle area, which is sufficient to allow for colonization without losing humidity in a birthed tub. If you use a larger tote, you need to consider passive air flow and potentially drill 2" holes slightly below where the handles are and cover them with a filter patch or lightly stuff them with polyfill to avoid restricting FAE. When your cake is fully colonized, you can "flip the lid" and introduce 12-hour light cycles from the sides of the tub. Alternatively, you can try what I call "shotgun tubs" where you clean another 6qt tub with isopropyl alcohol, let it dry, then spray it with preferably filtered or sterile water from the misting bottle and place it on top of the base tub with the lid removed. This allows light to come from above, which I've found helps with pinning formation, spread, and fruit growth direction; while aiding in moisture retention during the primordia formation and pinning initiation phases of the process (moisture is SO important in this hobby.
Generally 90% humidity or above within the enclosure at all stages of the process.
The more aspects of the process you can handle yourself, the cheaper the hobby becomes, and the easier it is to contain contamination and identify contaminants with greater reliability. I have never attempted a technique where you place a cake above water as it seems problematic and not feasible based on my personal techniques developed over the past few years. From what you mentioned, it seems like placing a colonized cake on moistened vermiculite within a plastic tote a few times larger than the cake itself might be a more appropriate approach for your project. However, don't let me deter you from experimentation! I have no judgments!
#mycology#myco#fungi#fungus#mushroom#gay#lgbt#enby#love#nature#beauty#art#fungusamoungus#mycojourney#mycochaotix#myco_chaotix#golden teacher#cubensi#mycelium#substrate#field capacity#q&a
6 notes
·
View notes
Text




Golden HaloX spore printing :) !!!
@mondayhares yes! Ty for saying so! I took these genetics out of a year cold storage just to get some fruits and make prints. I have another tub of them coming in for me to do golden swabs ^_^ haha. This variety is special for the golden brown spores! Many Cubensis usually will have black spore prints! The P. natalensis i posted in recent weeks are purple/black ^_^
#spore#spores#beauty#beautiful#art#gay#mycology#myco#mushroom#fungi#mycelium#mushies#mycochaos#magic mushies#nature#pscilocin#pscilocybin#paul stamets#pgt#cute#albino penis envy#ape#penis envy mushrooms
9 notes
·
View notes
Text





Penicillium on my plate, center is mushroom myc. Last few pics are after 1 and 2 minutes if pouring bleach on the plate!
#penicillin#penicillium#myco#mycology#mushroom#mycelia#mycelium#contam#fungus#fungi#mold#bleach#agar#gay#science#experiment#microbiology#microscope
6 notes
·
View notes
Text
#myco#mycology#contest#contamfam#mushroom#fungi#fungal#fungusamoungus#gay#lgbt#enby#mycelium#microscope#spore#spores#prize#prize bag
1 note
·
View note
Text

Drag queens and Trans people are NOT the problem for your sweet baby angel children’s innocence.
#gay#enby#trans#lgbt#churchpedo#catholic#religion#god#ridiculous#drag#queens#drag queen#transmasc#transfem
2 notes
·
View notes
Text


2 notes
·
View notes
Text




1 note
·
View note
Text




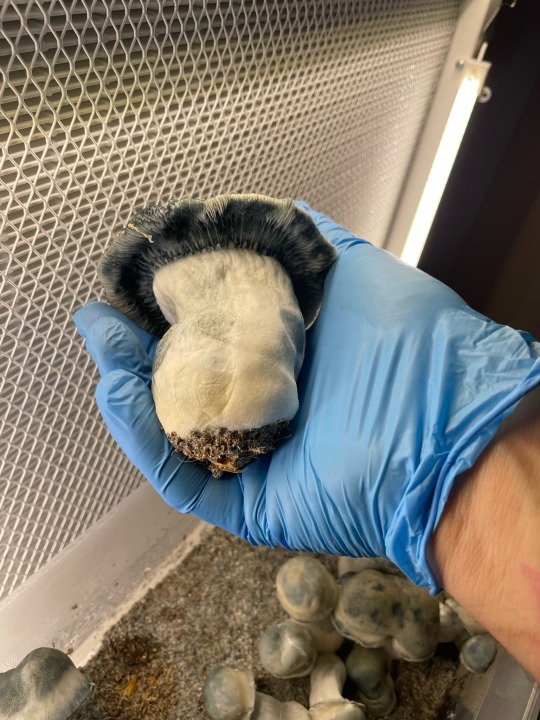

Albino penis envy :)
#mycology#myco#mushroom#gay#art#mushies#mycelium#mycochaos#fungi#magic mushies#nature#pscilocin#pscilocybin#paul stamets#pgt#beauty#cute#albino penis envy#ape#penis envy mushrooms
5 notes
·
View notes
Text

These APEs swoll 🍆🍆🍆
#ape#apes#albinopenisenvy#albino penis envy#mushroom#mycochaos#mycelia#mycelium#gay#art#mushies#fungi#magic mushies#mycology#myco#nature#pscilocin#pscilocybin#paul stamets#pgt#beauty#cute#penis envy mushrooms
9 notes
·
View notes
Text




Wild fungus mold! Unsure of species, but has similar morphologies to Trichoderma splattering ive seen in mushroom tubs. Growing amongst shitty quality backyard dirt, mixed with years of cig butts!
2 notes
·
View notes
Text
MycoChaotiX Q&A
Q: “can you explain how you mist once you see your first pins? thanks!”
A:
Greetings mycopal!Allow me to provide you with some additional information to fully address your question :)
Pinning is a process that occurs throughout the entire colonized cake, where the mycelium's metabolic processes shift towards fruiting. This is why, if someone's cake develops significant side pins, you often only see pinning there, rather than flat-surface pins through the fruiting phase. It's important to ensure that the colonizing mycelium is not exposed to the same lighting conditions as during fruiting. I personally keep black tarp material on my shelves to prevent light from hitting the bottom of the clear totes. However, light serves as a secondary trigger for pinning, while increased fresh air exchange (FAE), a drop in temperature, and the presence of condensation droplets on the mycelial mat (which later dry up) are the primary triggers for pinning after colonization is complete.
In my current setup using unmodified and modified 6qt shoebox tubs, I don't need to mist much. During colonization, I don't mist at all. The lid on the 6qt tubs, combined with the substrate's field capacity, provides enough moisture. You should notice notable condensation on all walls of the tubs throughout the entire process. The shoeboxes have built-in gaps in the lid-lock structure that allow for some passive FAE. These tubs were originally designed to allow air circulation for storing shoes (particularly for shoe collectors), but the passive FAE is sufficient to let excessive carbon dioxide escape while maintaining humidity around 90%.
When I observe full colonization of the subsurface sides of the tub and at least 80% of the tub surface covered in surface mycelium, I transition to the fruiting stage. My process has become quite straightforward: I remove the lid from the colonized tub and place a new shoebox tub (which may or may not be modified) upside down on top of it. Before placing the new tub, I mist it lightly. Then, I move the tub to a lower shelf in my grow and research area. The top level shelves are not exposed to my grow lights and are slightly shaded; and has a slightly lower ambient temperature. I also have a timed HEPA filter running on the lowest setting, cycling on and off every 6 hours. Additionally, I use brand: mostthink LED plant lights on the fruiting shelves.
This tub setup allows light to spread evenly over the substrate surface, through the new-tub into the colonized tub more effectively compared to fruiting with the lid on. If you choose to fruit with the lid on, you need to be mindful of the direction from which the light enters as the fruits can grow sideways. At this point, I still haven't misted the colonized substrate or the inner walls of the tub. The lips of the two shoebox tubs should align, but there can be a slight offset, which helps with passive FAE and allows intentional air-filtered flow for exchanging fresh air and removing carbon dioxide.
After a few days, I usually start to observe budding primordia as the increased FAE and air exchange in the tub cause the condensation on the mycelial mat to dry up. Once you see primordia, it becomes easy to spot their development from that point onwards. At this stage, I closely monitor the humidity in the tub. If I notice a lack of present and collecting condensation droplets on the inner walls of both the colonized and "roof" tub, I may use a light misting hand-pump spray bottle. I personally use a 250mL hair stylist mister bottle with filtered (or boiled and cooled) water, to which I add 1 tsp of H202. However, I never spray the mycelium or the fruits directly.
I MAY do a misted spray upwards, away from the colonized tub, and let it slowly sway and sink onto the substrate (but only if I havent begun to see primordia forming in that few day window I discussed above). I do this, primarily because of my belief/understanding that pins form on parts of the mycelial matte (generally rhizoid growth that have laid flat) and do so in spots where there was condensation pooled that dries from the FAE increasing :)
Does this help? Follow up questions? :)Thanks for reading!
#myco#mycology#mycochaotix#mushroom#fungi#misting#pins#primorida#mycopal#mycelium#mycelia#spore#spores#air filter#mostthink#fae
3 notes
·
View notes


