#Biomolecules
Text

Exploring RNA Interference
Imagine a molecular switch within your cells, one that can selectively turn off the production of specific proteins. This isn't science fiction; it's the power of RNA interference (RNAi), a groundbreaking biological process that has revolutionized our understanding of gene expression and holds immense potential for medicine and beyond.
The discovery of RNAi, like many scientific breakthroughs, was serendipitous. In the 1990s, Andrew Fire and Craig Mello were studying gene expression in the humble roundworm, Caenorhabditis elegans (a tiny worm). While injecting worms with DNA to study a specific gene, they observed an unexpected silencing effect - not just in the injected cells, but throughout the organism. This puzzling phenomenon, initially named "co-suppression," was later recognized as a universal mechanism: RNAi.
Their groundbreaking work, awarded the Nobel Prize in 2006, sparked a scientific revolution. Researchers delved deeper, unveiling the intricate choreography of RNAi. Double-stranded RNA molecules, the key players, bind to a protein complex called RISC (RNA-induced silencing complex). RISC, equipped with an "Argonaut" enzyme, acts as a molecular matchmaker, pairing the incoming RNA with its target messenger RNA (mRNA) - the blueprint for protein production. This recognition triggers the cleavage of the target mRNA, effectively silencing the corresponding gene.
So, how exactly does RNAi silence genes? Imagine a bustling factory where DNA blueprints are used to build protein machines. RNAi acts like a tiny conductor, wielding double-stranded RNA molecules as batons. These batons bind to specific messenger RNA (mRNA) molecules, the blueprints for proteins. Now comes the clever part: with the mRNA "marked," special molecular machines chop it up, effectively preventing protein production. This targeted silencing allows scientists to turn down the volume of specific genes, observing the resulting effects and understanding their roles in health and disease.
The intricate dance of RNAi involves several key players:dsRNA: The conductor, a long molecule with two complementary strands. Dicer: The technician, an enzyme that chops dsRNA into small interfering RNAs (siRNAs), about 20-25 nucleotides long. RNA-induced silencing complex (RISC): The ensemble, containing Argonaute proteins and the siRNA. Target mRNA: The specific "instrument" to be silenced, carrying the genetic instructions for protein synthesis.
The siRNA within RISC identifies and binds to the complementary sequence on the target mRNA. This binding triggers either:Direct cleavage: Argonaute acts like a molecular scissors, severing the mRNA, preventing protein production. Translation inhibition: RISC recruits other proteins that block ribosomes from translating the mRNA into a protein.
From Labs to Life: The Diverse Applications of RNAi
The ability to silence genes with high specificity unlocks various applications across different fields:
Unlocking Gene Function: Researchers use RNAi to study gene function in various organisms, from model systems like fruit flies to complex human cells. Silencing specific genes reveals their roles in development, disease, and other biological processes.
Therapeutic Potential: RNAi holds immense promise for treating various diseases. siRNA-based drugs are being developed to target genes involved in cancer, viral infections, neurodegenerative diseases, and more. Several clinical trials are underway, showcasing the potential for personalized medicine.
Crop Improvement: In agriculture, RNAi offers sustainable solutions for pest control and crop development. Silencing genes in insects can create pest-resistant crops, while altering plant genes can improve yield, nutritional value, and stress tolerance.
Beyond the Obvious: RNAi applications extend beyond these core areas. It's being explored for gene therapy, stem cell research, and functional genomics, pushing the boundaries of scientific exploration.
Despite its exciting potential, RNAi raises ethical concerns. Off-target effects, unintended silencing of non-target genes, and potential environmental risks need careful consideration. Open and responsible research, coupled with public discourse, is crucial to ensure we harness this powerful tool for good.
RNAi, a testament to biological elegance, has revolutionized our understanding of gene regulation and holds immense potential for transforming various fields. As advancements continue, the future of RNAi seems bright, promising to silence not just genes, but also diseases, food insecurity, and limitations in scientific exploration. The symphony of life, once thought unchangeable, now echoes with the possibility of fine-tuning its notes, thanks to the power of RNA interference.
#science sculpt#life science#science#molecular biology#biology#biotechnology#dna#double helix#genetics#artists on tumblr#rna#rna sequencing#RNA interference#cell biology#cells#biomolecules#illustrates#scientific illustration#illustration#illustrative art#scientific research
10 notes
·
View notes
Text
MAIN THAK GAYI HU BC IT’S A 45 MINUTE LECTURE I HAVE BEEN WRITING FOR TWO HOURS AND I’VE STILL NOT FINISHED IT AAAHHHH
#tw caps#desi academia#desi tag#studyblr#organic chemistry#biomolecules#pcm#jee#pcb#neet#india#engineeing#for context the teacher is just reading from the ppt and i have to make notes
22 notes
·
View notes
Text
Shedding new light on sugars, the “dark matter” of cellular biology - Technology Org
New Post has been published on https://thedigitalinsider.com/shedding-new-light-on-sugars-the-dark-matter-of-cellular-biology-technology-org/
Shedding new light on sugars, the “dark matter” of cellular biology - Technology Org
Scientists at Université de Montréal’s Department of Chemistry have developed a new fluorogenic probe that can be used to detect and study interactions between two families of biomolecules essential to life: sugars and proteins.
Our idea was to label sugar molecules with a chromophore, a chemical that gives a molecule its colour,” explained Cecioni. “The chromophore is actually fluorogenic, which means that it can become fluorescent if the binding of sugar with the lectin is efficiently captured. Image credit: Cecioni Lab
The findings by professor Samy Cecioni and his students, which open the door to a wide range of applications, were published in mid-October in the prestigious European journal Angewandte Chemie.
Found in all living cells
Sugar is omnipresent in our lives, present in almost all the foods we eat. But the importance of these simple carbohydrates extends far beyond tasty desserts. Sugars are vital to virtually all biological processes in living organisms and there is a vast diversity of naturally occurring sugar molecules.
“All of the cells that make up living organisms are covered in a layer of sugar-based molecules known as glycans,” said Cecioni. “Sugars are therefore on the front line of almost all physiological processes and play a fundamental role in maintaining health and preventing disease.”
“For a long time,” he added, “scientists believed that the complex sugars found on the surface of cells were simply decorative. But we now know that these sugars interact with many other types of molecules, particularly lectins, a large family of proteins.”
Driving disease, from flu to cancer
Like sugars, lectins are found in all living organisms. These proteins have the unique ability to recognize and temporarily attach themselves to sugars. Such interactions occur in many biological processes, such as during the immune response triggered by an infection.
Lectins are attracting a lot of attention these days. This is because scientists have discovered that the phenomenon of lectins “sticking” to sugars plays a key role in the appearance of numerous diseases.
“The more we study the interactions between sugars and lectins, the more we realize how important they are in disease processes,” said Cecioni. “Studies have shown how such interactions are involved in bacteria colonizing our lungs, viruses invading our cells, even cancer cells tricking our immune system into thinking they’re healthy cells.”
Difficult to detect…until now
There are still many missing pieces in the puzzle of how interactions between sugars and lectins unfold because they are so difficult to study. This is because these interactions are transient and weak, making detection a real challenge.
Two of Cecioni’s students, master’s candidate Cécile Bousch and Ph.D. candidate Brandon Vreulz, had the idea of using light to detect these interactions. The three researchers set to work to create a sort of chemical probe capable of “freezing” the meeting between sugar and lectin and making it visible through fluorescence.
The interaction between sugar and lectin can be described using a “lock and key” relationship, where the “key” is the sugar and the “lock” is the lectin. Chemists have already created molecules capable of blocking this lock-and-key interaction, and can now to identify exactly what sugars are binding to lectins of high interest to human health.
“Our idea was to label sugar molecules with a chromophore, a chemical that gives a molecule its colour,” explained Cecioni. “The chromophore is actually fluorogenic, which means that it can become fluorescent if the binding of sugar with the lectin is efficiently captured. Scientists can then study the mechanisms underlying these interactions and the disturbances that can arise.”
Cecioni and his students are confident their technique can be used with other types of molecules. It may even be possible to control the colour of new fluorescently labelled probes that are created.
By making it possible to visualize interactions between molecules, this discovery is giving researchers a valuable new tool for studying biological interactions, many of which are critical to human health.
Source: University of Montreal
You can offer your link to a page which is relevant to the topic of this post.
#applications#Bacteria#Biology#Biomolecules#Cancer#cancer cells#Cells#cellular structures#challenge#chemical#chemistry#Chemistry & materials science news#Dark#dark matter#detection#Disease#Diseases#diversity#Explained#Fundamental#Giving#Health#how#human#Human health#immune response#immune system#infection#interaction#it
2 notes
·
View notes
Text
youtube
What is Cryogenic Transmission Electron Microscopy or Cryo-TEM?. This is an introductory lecture about Cryogenic Transmission Electron Microscopy (Cryo-TEM) to the interdisciplinary audience. Topics including, conventional TEM imaging, negative staining, supercritical drying, and single particle reconstruction are covered in this lecture.
#microscope#microscopy#image#nano#nanoparticles#nanotechnology#virus#3dimages#electronmicroscope#electronmicroscopy#colloids#negative staining#vitrification#transmission#protein#biomolecule#biomolecules#electronbeam#chemistry#structure#vaccines#vaccine#cell#biology#structuralengineering#structuralanalysis#crystals#crystallography#hydrogenbond#supercriticaldrying
3 notes
·
View notes
Text
The Four Vital Macromolecules of Biology: Structure, Differences, and Functions
Biology is an intricate tapestry of interactions and functions, with the fundamental building blocks being macromolecules. These large molecules play significant roles in almost all biological functions and processes. They are crucial for the structure, function, and regulation of the body’s tissues and organs. Let’s delve into the four primary macromolecules, exploring their differences and…

View On WordPress
2 notes
·
View notes
Text
Lupine Publishers | Palauamine and Olympiadane Nano Molecules Incorporation into the Nano Polymeric Matrix (NPM) by Immersion of the Nano Polymeric Modified Electrode (NPME) as Molecular Enzymes and Drug Targets for Human Cancer Cells, Tissues and Tumors Treatment under Synchrotron and Synchrocyclotron Radiations

Editorial
In the current editorial, we study Palau’amine and Olympiadane Nano molecules (Figures 1 & 2) incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations. In this regard, the development of Chemical Modified Electrodes (CEMs) is at present an area of great interest. CEMs can be divided broadly into two main categories; namely, surface modified and bulk modified electrodes. Methods of surface modification include adsorption, covalent bonding, attachment of polymer Nano films, etc. Polymer Nano film coated electrodes can be differentiated from other modification methods such as adsorption and covalent bonding in that they usually involve multilayer as opposed to monolayer frequently encountered for the latter methods. The thicker Nano films imply more active sites which lead to larger analytical signals. This advantage coupled with other, their versatility and wide applicability, makes polymer Nano film modified electrodes particularly suitable for analytical applications [1–27].
Electrochemical polymerization offers the advantage of reproducible deposition in terms of Nano film thickness and loading, making the immobilization procedure of a metal–based electro catalyst very simple and reliable for Palau’ amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations. Also, it must be notice that the nature of working electrode substrate in electro preparation of polymeric Nano film is very important, because properties of polymeric Nano films depend on the working electrode anti–cancer Nano materials. The ease and fast preparation and of obtaining a new reproducible surface, the low residual current, porous surface and low cost of Multi–Walled Carbon Nanotubes (MWCNTs) paste are some advantages of Carbon Paste Electrode (CPE) over all other solid electrodes [28–92].
On the other hand, it has been shown that, macrocyclic complexes of Palau’amine and Olympiadane Nano molecules– encapsulating Carbon nanotubes are interest as modifying agents because in basic media Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes redox centers show high catalytic activity towards the oxidation of small organic anti-cancer Nano compounds. The high–valence species of Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes seem to act as strong oxidizing agents for low-electroactivity organic substrates. 1,2–Dioxetane (1,2– Dioxacyclobutane), 1,3–Dioxetane (1,3– Dioxacyclobutane), DMDM Hydantoin and Sulphobe as the anti–cancer organic intermediate products of methanol oxidation as well as formic acid, is important to investigate its electrochemical oxidation behavior in Palau’ amine and Olympiadane Nano molecules-encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations [93–110].
In this editorial, we decided to combine the above mentioned advantageous features for the aim of Palau’ amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations. Furthermore, in this editorial, we prepared poly Nano films by electropolymerization at the surface of Multi-Walled Carbon Nanotubes (MWCNTs) paste electrode. Then, Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes were incorporated into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) in a solution. The modifier layer of Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes at the electrode surface acts as a Nano catalyst for the treatment of human cancer cells, tissues and tumors under synchrotron and synchrocyclotron radiations. Suitability of this Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes–modified polymeric Multi–Walled Carbon Nano tubes (MWCNTs) paste electrode toward the electrocatalytic treatment of human cancer cells, tissues and tumors under synchrotron and synchrocyclotron radiations in alkaline medium at ambient temperature was investigated [111– 153].
For more information about Archive of Organic and Inorganic Chemical Sciences please click https://lupinepublishers.com/chemistry-journal/
For more Lupine Publishers please click on below link
https://lupinepublishers.com/index.php
#lupine#lupine publishers#archive of organic and inorganic chemical sciences#organic compounds#biomolecules#polymer chemistry#cluster compounds#chemical biology#mechanism of action#xenobiotic metabolism#lupine publishers llc#open access journals
4 notes
·
View notes
Text
log #10
life is a bit like denaturing a protein- sometimes you just have to cut out what's making you spiral
#pick up lines#biology#biomolecules#biochemistry#this only works for protein's secondary structure btw#specifically the a helix#or maybe it works if you really think about it#in the tertiary structure
3 notes
·
View notes
Text
Magic of Biomolecules: Building Blocks of Life in Your Soil
In the intricate realm of soil science, an often-overlooked marvel plays a pivotal role – Biomolecules. These minuscule powerhouses operate quietly beneath the surface, influencing the soil's health and, consequently, the prosperity of crops. Let's explore the domain of Biomolecules, delving into their nature, functions, and significance in the agricultural landscape.
1 note
·
View note
Photo
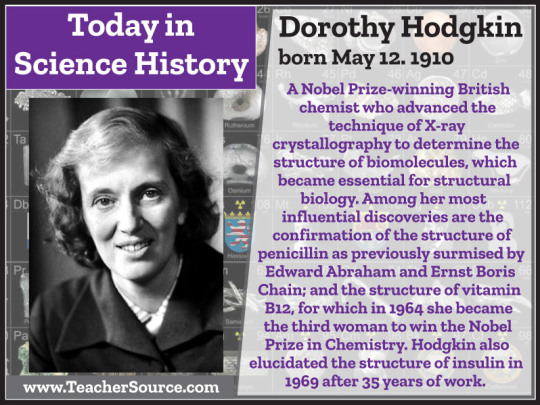
Dorothy Hodgkin was born on May 12, 1910. A Nobel Prize-winning British chemist who advanced the technique of X-ray crystallography to determine the structure of biomolecules, which became essential for structural biology. Among her most influential discoveries are the confirmation of the structure of penicillin as previously surmised by Edward Abraham and Ernst Boris Chain; and the structure of vitamin B12, for which in 1964 she became the third woman to win the Nobel Prize in Chemistry. Hodgkin also elucidated the structure of insulin in 1969 after 35 years of work.
#dorothy hodgkin#x-rays#biomolecules#biology#structural biology#penicillin#nobel prize#nobel prize winners#women in science#women in history#science#science history#science birthdays#on this day#on this day in science history
1 note
·
View note
Link
NCERT solutions for class 12 Chemistry chapter 14 Biomolecules, including thorough solutions for reference. These solutions are updated according to the latest term – II CBSE syllabus for 2021-22 and are provided in easy language for understanding. Tips and tricks are also provided.
0 notes
Text
This Diwali, I finally started Biomolecules ✨
3 notes
·
View notes
Text
Scientists Train AI to Make More of Life’s Building Blocks - Technology Org
New Post has been published on https://thedigitalinsider.com/scientists-train-ai-to-make-more-of-lifes-building-blocks-technology-org/
Scientists Train AI to Make More of Life’s Building Blocks - Technology Org
A new research paper in Science details an artificial intelligence (AI) upgrade that significantly enhances scientists’ ability to model and generate biomolecules, the building blocks of life.
Recent breakthroughs in AI now allow scientists to create protein molecules unlike any found in nature. Image credit: Ian Haydon, UW Medicine Institute for Protein Design
This breakthrough is the work of academic researchers at the Institute for Protein Design at the University of Washington School of Medicine, who have made their new tools freely accessible to the scientific community.
In the rapidly evolving field of AI-driven science, this advance builds upon the success of AlphaFold (a tool from Google DeepMind), and RoseTTAFold and RFdiffusion (both developed at the institute).
The paper’s lead authors are postdoctoral scholar Jue Wang and graduate students Rohith Krishna and Woody Ahern — all members of David Baker’s lab. Baker is a professor of biochemistry at UW Medicine and director of the Institute for Protein Design.
In the new study, the scientists first retrained the protein modeling tool RoseTTAFold so it could accurately model how proteins interact with common molecules found in living cells such as DNA, RNA, metal ions, sugars and other small chemicals.
The team named their new tool RoseTTAFold All-Atom, reasoning that a single AI model trained on data from all the major types of biomolecules would become a useful tool for life sciences research. In the paper, the researchers show that RoseTTAFold All-Atom can predict in detail how particular proteins and DNA stretches interact, how certain drug molecules may bind to human receptors, and more.
“We made RoseTTAFold All-Atom free so that scientists everywhere can make new discoveries about the molecules that run all of biology. It may enable them to understand the molecular mechanisms of many diseases better, and this may unlock new treatments,” said Krishna.
The team then used their upgraded AI model to enhance RFdiffusion, a widely used generative AI system that can create proteins unlike any found in nature. Lab tests confirmed that RFdiffusion All-Atom can generate proteins with pockets that bind to specific compounds, including the steroid digoxigenin, the iron-rich blood molecule heme, and chemicals used by plants to absorb sunlight.
This demonstrates that AI can generate a wide variety of advanced biological functions.
“Our goal here was to build an AI tool that could generate more sophisticated therapies and other useful molecules. For instance, researchers can now design proteins that shut down specific disease-causing molecules, paving the way for precise and effective treatments,” Ahern said.
“By empowering scientists everywhere to generate biomolecules with unprecedented precision, we’re opening the door to groundbreaking discoveries and practical applications that will shape the future of medicine, materials science, and beyond,” Baker said.
Source: University of Washington
You can offer your link to a page which is relevant to the topic of this post.
#A.I. & Neural Networks news#ai#ai model#AlphaFold#applications#artificial#Artificial Intelligence#artificial intelligence (AI)#atom#biochemistry#Biology#Biomolecules#Biotechnology news#blood#Building#Cells#chemical synthesis#chemicals#Chemistry & materials science news#Community#data#DeepMind#Design#details#Discoveries#Disease#Diseases#DNA#drug#Future
0 notes
Text
had my version of drunk history tonight when someone w no science background wanted a detailed description of my research whilst i was several drinks in
#he was surprised bacteria have complex things like proteins so i was trying to explain that there are 4 biomolecules everyones got#(carbohydrates lipids nucleic acids proteins)#and it says something about my level of sobriety that i was struggling to list the four for him#this should be my test of handling alcohol in future. how many drinks until you forget the central dogma of molec bio or something
8 notes
·
View notes
Text

Se esce qualcosa di NuovoOlimpo esco dall'aula
3 notes
·
View notes
Text
log #3
i think we're a ß 1-4 glycosidic linkage- because through ups and downs, we'll stick together anyway.
0 notes
Text
i cannot believe that kyle drops a sewonso vid literally five minutes before i have a three hour lab and then an exam
#i was not emotionally prepared !! n now i can’t even brainrot bc my head is too full w chem n biomolecules n shit#kyle u dick#where was the warning. where#blessed.txt
6 notes
·
View notes