#ph indicator
Text
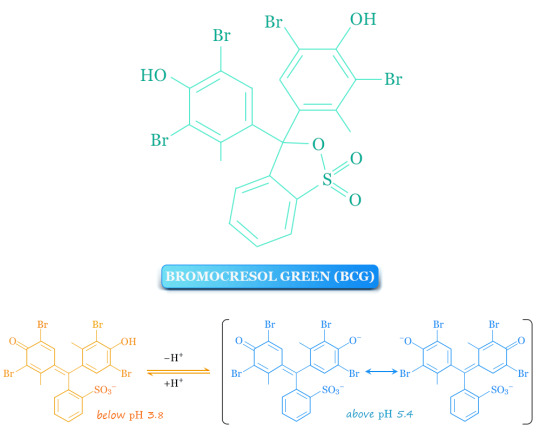
Bromocresol green (BCG) is a dye that is used in chemistry and analytical chemistry as a pH indicator in acid-base titration and in thin-layer chromatography staining solutions to visualize acidic compounds. Generally, the indicator can be prepared by dissolving BCG in an ethanol solution.
#Bromocresol Green#Bromocresol#phenolphthalein indicator#ph indicator#chemistry#analytical chemistry
5 notes
·
View notes
Text

Red & Blue Litmus Paper Acid/Base pH Indicator Strips
Comes with 2 portable vials, each filled with easy to use, pre-cut strips
No external reagents or chemicals needed for accurate testing of solutions
A simple way to determine without a doubt if a solution is acidic or basic, with a second test to confirm your findings
No color chart needed to determine findings; these tests are designed for determining if the pH is above or below a certain level NOT giving a precise pH value
PROUDLY made in America
More info click here>>
1 note
·
View note
Text
I've been meaning to make more posts about this, but there's so much i have to say/images to show that this will be a part 1 out of ??? and this one focuses mainly on the first batch of Purple Iris watercolors and the pH mystery they made me unravel through chaotically-organized researching.
Basically, I've been messing around with anthocyanins, a common class of plant pigments that are pH sensitive/can be used as a pH indicator. The first source I've tried has been purple irises, which i've only vaguely been familiar with in the past. The ones I picked were the ones that had begun to shrivel slightly, to the point where they were still a deep purple but picking them they would almost be leaking a purple liquid that stained my hands. I put them in a thing of hot tap water (not boiled, just the hot setting on the faucet), enough to cover the flowers, and let them steep. they began changing the color of the water almost immediately, with the fresher ones not losing their color as quickly as the ones that had begun to wilt on the plant. within 30 minutes i decided it was extracted enough.

This left a strong purple in the water, which i then poured off into three other containers, two of which i would alter the pH of.
The purple is due to delphinidin, a type of anthocyanidin that forms the building blocks of anthocyanins. Note i italicize the word anthocyanidin just so it's easier to tell apart the two.
there are anywhere from 16-31 anthocyanidins depending on what source you find, but they are basically the backbone structure of anthocyanins, of which there are over 600 something. The main thing that turns an anthocyanidin (aglycon) into an anthocyanin (glycoside form) is a sugar attached to it.
Realistically, that distinction isn't useful when simply extracting things from flowers in hot water, but i thought it was a fun fact to note. Anthocyanidins also come in handy for knowing what builds the anthocyanins in your flowers/plant part;
cyanidin (30%), delphinidin (22%), and pelargonidin (18%) make up the base for a good majority of all the anthocyanins in plants (~60% collectively),
peonidin, malvidin, and petunidin being runnerups (20% collectively)
the 20-something remaining anthocyanidins make up the rest
So basically, they all have slightly different colors that are pH reactive, and can provide anything from red to pink to orange to purple to blue. But, for our purposes, if you have a blue/purple flower, that likely means it has some amount of delphinidin-based anthocyanins in it! there can also be more than one anthocyanidin type present in the same plant.
Other well-known sources of anthocyanins are grape skins, red cabbage, red onions, butterfly pea tea, and purple violets. However, they're also very abundant in many many other plants, these are just the common ones i can think of that lots of people are probably familiar with to some degree.
Fun fact, grape skins are actually really well-studied as far as anthocyanins go (i believe they mainly have malvidin-based ones) because they're so important for the coloration of wine!
Anthocyanins as a whole are also studied as a natural source of food dyes, along with other flavonoids such as carotenoids.
As for why it turns colors, this is because of the way the anthocyanin changes structure in different pHs. The short answer is it turns red/pink in low pH (acidic) conditions, purple in slightly acidic/neutral conditions, and blue/green in slightly high pH conditions.
The long answer is something I'll explain in a moment, but for now here's the acid/base colors:


(on the left, i altered it with vinegar and it became a bright magenta color; on the right, i altered it with baking soda and it became a sea green/blue cyan that refused to show up accurately on camera). That's one thing I've noticed, and others have too, is that when working with pigments (especially natural ones) the color accuracy of the camera often just completely fails. there's only so many colors a digital camera can capture!
Here's a slightly more accurate color due to different lighting, note how it's more a malachite green than a pure blue. off the bat this was interesting becuase I wasn't expecting as green of a liquid as i got.

Anyways, the first thing i did with them was use them as-is, no alterations past the addition of the respective vinegar and baking soda. I painted with them just as one would paint with watercolors, and interestingly enough, when i put them onto paper, they began to change from their pink/purple/malachite colors to a teal/indigo/emerald set instead.
This seems to be the result of something in the paper itself, likely calcium carbonate (which i only recently learned is added to "buffer" paper against acidic substances; the cellulose in paper is more stable long term when there's no acid present, and the calcium carbonate neutralizes any acids applied to a degree).
It's still interesting that even though the acids are neutralized, they give a unique color when compared to the basic paint.
I also tried soaking some of the same paper in vinegar water, which got rid of that buffer and let me paint with the pinks intact, but that's for another post.
Also, note that i said "neutral" for the middle, this is just what i wrote down for the tap water sample; in actuality the tap water is actually a bit closer to pH 6 instead of a true neutral 7, which i only found out after i had gotten this far. So whenever i say "neutral," i mean "tap water that's slightly acidic"
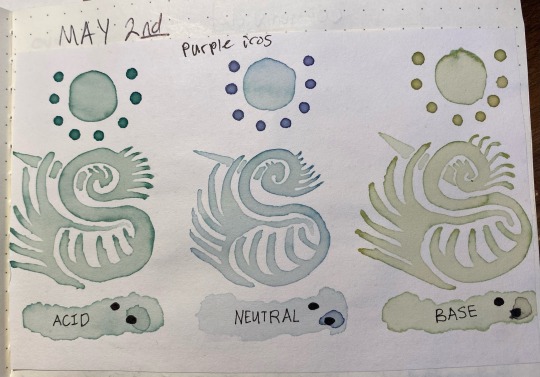
Here's something interesting that happened, though. Overnight i left the jars on my desk, and while the acid and neutral colors were the same when I came back ~24 hours later, the basic had degraded into a murky brown. this was interesting since that meant the instability was pH-dependent.
So, i made another color swatch with the acid/neutral/base, and used that as a comparison to look at how it had changed. Surprisingly, it painted out a yellowy-green instead of a murky gray-brown
here's the murky water that the once-malachite-green turned into:

I also poured off a bit into another container, and shifted it back into a low pH with a bit of vinegar to see if it would still change color. Surprisingly, it turned a slight pink, like pink lemonade, which means there were still anthocyanins in there but they were likely a lot less concentrated than they used to be.
Here's the pink, with a few leftover bubbles from the baking soda/vinegar reaction:

Here's the results of painting with these:
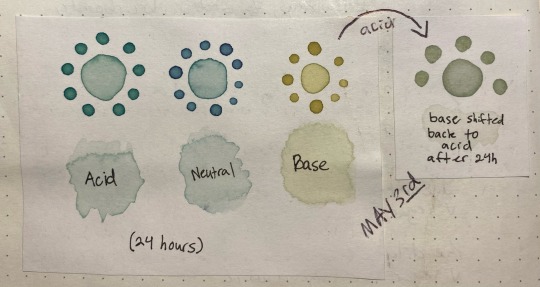
the acid was basically unchanged after sitting in a jar for 24 hours, the neutral had lost a bit of its purple color but was still about the same, and the base was now a lot yellower/tanner with a bit of green still showing through. The shifted sample was a pale stormy gray that ended up taking on a green color as it dried, as though following the trend of pink shifting to a bluer color but on a much more muted scale.
Now obviously, i wanted to figure out what caused this, so i dug around both on wikipedia and other sites but found myself eventually reading into scientific papers on the topic, at which point it became very clear that i would need to learn like, organic chemistry and such to be able to say for sure what was happening.
I did eventually manage to figure out a few things despite the dense terminology; for one thing, anthocyanins are more unstable than other plant pigments such as carotenoids. there are plenty of things that can affect their stability, including the pH of the substance they're stored in. Any higher than pH 7 (basic pHs) and theyll begin to degrade. This explains why the high pH sample lost its blue/green color, and why there was very little left to be shifted back to a pink color.
I also found out that the pH color shift isn't as simple as it seemed. Rather, there are multiple chemical forms of anthocyanins.
At the lowest pHs, basically all of them are in the "flavylium cation" state, which basically means it's positively charged and this is what gives a red color
still at a low pH (2-4), there's anothe chemical form that appears, the "quinoidal" structure that gives a blue color. Note that the red cation is still present, just no longer the only form
the more the pH rises, the more forms start to coexist, with some of those forms being colorless (one of which is a "colorless carbinol"
so, between 4 and 6 there are the cations (red) quinoidal (blue) carbinol (colorless) and something called a chalcone that gives a pale yellow
and then past that, I'm unsure, but of course that's around when the anthocyanins begin to degrade
There are also a lot more than these that i've encountered in various contexts but these seem to be the basic ones.
Do note that i do not fully understand these terms (flavylium, quinoidal, carbinol, chalcone, etc.) and have only recently begun to actually try to learn what they mean and the context surrounding them as i only had a class of basic high school chemistry under my belt prior to this. The main paper i combed over to try to find info on it seems to be behind a paywall but the DOI is:
doi.org/10.1016/j.foodchem.2008.09.001
for anyone curious and able to access it, whether through legit means or what have you.
That being said, to me, the takeaway here seems to be that there's a yellow form that appears around the time that other color forms begin to disappear, and as those degrade it makes sense that the resulting degraded forms also contribute to a murky color. This helps explain why it changed color in the jar and also retained a bit of yellow and green.
This also explains why the blue form seems to also be slightly green, it's got the blue quinoidal chemical form as well as the yellow chalcones.
There are also interesting things of note that I will get into at a later date, such as the texture/reflectivity of the way it dries, the differences in extraction ease between this and purple violets, the addition of a genuinely neutral/pH 7 sample later, a sample from a plant that doesnt seem to have delphinidins, and sample the seems to genuinely sparkle??? Much more of interest to come soon!
#art#traditional art#anthocyanins#watercolors#purple irises#painting#science#natural dyes#pigments#dyes#pH indicators
61 notes
·
View notes
Text

My dog Ruby (right) met the Grinch.
#dogs#submission#LOVE how you added the direction indicator for your pet.#could not tell who was who until i read the caption#ph
119 notes
·
View notes
Text
also i have no merch of prompto ffxv but i still love him so much. its been like. Its going to be Holy fuck.. 8 years in like september. whaat the fuck theres no way. number one fictional guy of all time to me. Number two actually. shadow the hedgehog is cooler. i had a crush on him when i was 9 i think.
Whatever im going to bed i have work in the morning ^_^ setting up samples of blue and also um. making standards for the data collection. whee! goodnight lol 💜
#astr.txt#Google is toluidine blue a pH indicator#also i dont talk much about selfship because im shy about it ^^; sourry
4 notes
·
View notes
Text
One thing I have been amused to learn poring over various Renaissance-era medical herbals is that borage -- one of my very favorite flowers, for a number of reasons -- was once considered one of the better "cures" for melancholy
15 notes
·
View notes
Note
for the 2 + 3 = 5 for Jade and Azul with the words perfume, tea, and purple?
2 characters + 3 words = 5 sentences
Azul peered suspiciously at the steaming cup of unusually deep blue tea that Jade placed on the corner of his desk.
The tall moray gestured to it, smiling benignly, and said, "This is the item that I suggested we add to the lounge menu, Azul; please squeeze in the lemon and observe."
Azul raised an elegant eyebrow, but complied. His other eyebrow raised to match the first as he watched the liquid in the teacup go from blue to purple.
Jade went on, "It's frightfully popular due to the color change effect, although it's a real shame that the unsweetened tea tastes like drinking perfume."
#butterfly pea flower tea is a natural ph indicator and turns the prettiest purple when lemon or lime juice is added#the taste can best be described as 'well i can drink it'#thanks for the ask!#azul ashengrotto#jade leech#2 characters + 3 words
6 notes
·
View notes
Text
Oppenheimer spoilers below
Christopher Nolan.

You done it again.
I’m sure I could touch on so many things in this movie. I might later, I might not. But leaving the theater I felt so deeply unsettled, and I wanted to touch on that, from my point of view as an amateur writer and someone starting to feel comfortable talking about cinematography (although I have no current plans to do much more than talk about it).
Now I’d like to start by saying that, if you haven’t seen it or it didn’t make that big of an impact on you, this is a long movie. I, myself, am no stranger to long movies and I think it was marvelously done. That being said, my memory tends to leave things to be desired, so while I am writing this rather soon after I watched and digested it, others may have stronger points/counter points/evidence/what-have-you.
With that out of the way, I’d like to talk about one facet of the movie that Christopher Nolan, imo, harnessed in a way that I did not expect. And it wasn’t color, it wasn’t camera perspective, it wasn’t even the timeline.
It was his usage of sound.
From the very beginning, sound has always been a key factor in this movie. In the trailer, the crackling of the radiation detector is ominously present. It is used less frequently in the movie than I had predicted, but when it makes its “appearance” it most certainly put me on the edge of my seat. It brings a sense of gravity to an already serious situation. Engrossed in the movie as I was, I admit the sound mainly made me uneasy because it demonstrated the presence of radiation. Looking back, I can add to that and say it may have also been used as a foreshadowing tool.
Now, I’m sure we’ve all heard sound used a foreshadowing tool. The little girl screaming for help is actually the protagonist with a shadowy past who can’t get the sound of the daughter/random child he couldn’t save out of his head. The words of an interrogation in the beginning of a movie finally get context half-way through when the timelines finally align. It’s been used, it’s been subverted, it’s nothing new.
Except when it is. In my experience, and I am the first to admit that I haven’t seen enough movies of the genre to have a definitive say in the matter, I have never been quite as unsettled or shocked by the background noises than while watching Oppenheimer.
Let me start with the sound that will not leave my mind. Those damned boots. I’d heard it at school pep rallys when everyone would stomp in the bleachers. I had never expected to hear it in a movie about the man who made the atomic bomb. When I first heard it, I thought it was an aesthetic choice, like picking the music to evoke a certain emotion. And while it is that, all of sound is, I never actually expected for it to be from such a central scene.
When the boots were first connected to a scene, a short, split-second, blink-and-you-miss-it shot of shoes-on-bleachers, it was early enough in the movie that I thought it was a flashback of a pep rally at one of his schools, maybe as a boy genius, and I let it go. Later on, when they show the full scene, it’s terrifying.
And then you can’t hear the boots at all.
For hours, all you could hear were those boots in the back of stressful scenes and now that they’re there, now that you can see them, suddenly they’re gone. You know what they’re supposed to sound like, so why would you need to hear them again? And it helps build the suspension, the tension that Oppenheimer is feeling during that scene. And so the next time you hear it in the movie, the next time those boots are stomping on the bleacher in the background when they are in a meeting or an interrogation, it pulls you right back into the stress and horror of the bleacher speech.
And then, of course, you begin to realize that while the timelines had been so well interwoven that it seemed like you couldn’t go two scenes without hearing those boots, it was always a very specific scene, a specific timeline that the boots would make an appearance. Because, of course, in one Oppenheimer hadn’t heard them yet, and in another it’s not Oppenheimer at all.
Another prominent sound that I mentioned earlier is the Geiger Counter (I finally looked up the name!). This is a foreshadowing of a different kind, more physically consequential than mental. This, I believe, foreshadows the heavy losses suffered later in the movie by the radiation poisoning. The blast itself only killed so many people, it was the radiation, as they are so fond of pointing out in the movie, that helped round out the total killed in the bombings.
I’ll admit that it’s a bit of a stretch, but I can see it being a small detail that was added in.
On another note, I’d like to address how Nolan also utilized the absence of sound.
Oppenheimer (movie) doesn’t necessarily have jump scares, per se, but rather I jumped a lot during the movie. More startled with a side of deep-seated dread rather than scared. Any way you put it, Nolan does a very good job of keeping me at the edge of my seat. I’ve mentioned earlier the lack of sound during the bleacher scene (containing about half of the many times I jumped during the movie) so I won’t go into that again.
Another part of the movie where he employs this is the bomb test. This is the culmination of years of hard work, the pinnacle of one of three (four?) timelines. And you can’t hear a thing. You get the countdown (a staple from the trailer), the drop, and then… nothing.
It’s a beautiful flash of light and an explosion but the whole time there’s not a sound to be heard. At first it feels like it’s gone just so you can look at the view and then as you’re lulled into security… the shockwave hits.
I can’t remember the last time I jumped so high in a movie theater.
And it’s used for every time you see the shockwave. Silence, wind, and then a force pushes everything back, rattling the house, whipping up the dust. It’s really iconic imo.
Anyway, I walked out fixated on the noises of that movie. And the cackling of the neon did not help my dazed state.
#oppenheimer#oppenheimer spoilers#spoilers for oppenheimer#I of course say all these things as a jealous writer who can only utilize these faculties if I find a way to write them down#as well as a starry-eyed movie-watcher#tbh the beginning of the movie was a little confusing for me#But I blame that on the fact that I hadn’t seen it before#Definitely one of those re-watch movies i think#Speaking of sound#I would love to see a psychologist/therapist go in depth#on whether or not Nolan’s usage of sound/light/hallucinations were indicative of Oppenheimer’s state of mind#and whether or not it was a symptom of something like PTSD#Or simply an artistic choice that was badly informed etc etc#very curious#if there is a video or article out there about it I would love to see it!!!#Kiki does movie reviews in the tags#Sorry I didn’t really mean to sound like a movie review when I wrote this but it just sort of came out like that#\ (ツ) /#anyway it was a really well done movie#The acting was ph en om en al#and definitely gave me an existential crisis at least once#would see again#and so should you#I may add more to this#but for now#adieu#just yelling into the void
6 notes
·
View notes
Note
When Tetra was petrified, she remained somewhat concious and could vaguely hear her surroundings, but, obviously, couldn't communicate back. Now, being a descendant of the royal bloodline and/or having a Triforce-induced superpowers, she'd usually be able to contact Link telepathically, but, after being turned to stone, Tetra's call is too quiet, and Link can't hear it, because he has his own innate and pretty potent wind magic, drowning any weak external signals, not to mention Ciela and her siblings adding their magic into the mix.
There is someone else who can hear her in that state, though, and that's Linebeck. He doesn't wield any magic (or nothing significant, at least), so he doesn't drown her out. Whenever Link and Ciela/other spirits leave his ship to go dungeon-crawling or stuff, if the engine's turned off, only then can Linebeck hear Tetra calling out. At first, he's naturally freaked out and thinks Link brougt a ghost from the Ghost Ship, but later they figure everything out and manage to communicate with Tetra and relay what she had to say to Link.
(Also, for a first couple of days Linebeck referred to Tetra as a "figuredeck", until realizing that the subject is too sensitive for Link).
That's a cool idea! Giving Tetra a sort of role, a new role for Linebeck, and some new group dynamic stuff, that's a neat concept.
#asks#goopi-e#loz#legend of zelda#phantom hourglass#this is a cool idea#giving tetra something to do during ph#giving her an actual role to some degree#i dont have a whole lot to say about this! since oyu kinda just phrased it as a statement rather than a question#so i can really process it as a point of discussion without more indication from you#but this is a really cool idea#ive personally just stuck to tetra being. frozen and like. asleep (i dont really. have an attachment to tetra so uh. idk)#also with how nebulous magic is in loz the idea of most characters having some amount of innate magic is cool#that idea has been cool to me#and ive personally leaned into linebeck having like. in rpg terms low mana high magical power.#like in my peus fic hes noted as having the ability to resurrect a dead person but only if certain conditions are met#the idea of him having to be the one tetra has to go through to talk to link also adds a bit of. tension? in my mind#since im set in viewing linebeck as low empathy and socially unsure and all of that so it evokes an idea of him. fucking it up a lot#or being highly uncomfortable or insensitive#what is a figuredeck im begging i looked it up and cant figure it out#sorry it took so long for me to write such a short answer my shitfucked brain forced me to spend 13 straight hours on legos#happy holidays btw hope things have been good with you#also again sorry if this is like. a letdown answer until i got the second ask i deadass thought this was a mistake sorry
7 notes
·
View notes
Text
People on reddit, tying themselves into knots as they try to explain why Aviation cocktails invariably look terrible in color: It’s supposed to resemble stormy skies! Or the aluminum grey of airplanes back then!
Me: My guys, it’s literally because modern crème de violettes are made with extra (often artificial) colors that mix badly with just about everything else. That’s it. That’s the reason.
#i still maintain my hot take that they're supposed to be pink not blue (or purple or grey or whatever) because guess what?#natural violet dye is a pH indicator!#alcohol#personal#i am still lacking in karma so i can't (yet) shout it in a post there so i have to shout it here sorry#anyway yes i bought a rothman & winter crème de violette (one of the oft-recommended ''good'' ones on the market)#and it's fucking terrible#it's OKAY in an aviation but only because it has other flavors to (mostly) cover it up#it looks and tastes artificial as shit and i had some straight and it had THE NASTIEST aftertaste bleh
2 notes
·
View notes
Text
whether or not you get excited when toilet paper is on sale is an excellent litmus paper test for being an Adult™️
#more accurately it's like a pH indicator with two colour changes because presumably at a certain point it becomes less exciting again#but that felt too sciency for the punch line#anyways i'm thrilled right now. bought 2 packs!!
2 notes
·
View notes
Text
OH YEAH I made wild violet tea !! no picture of the finished product yet but


[ID: Two images and one video. The first image is a photo of a bunch of purple wild violets in a small metal strainer, sitting on a plaid dishcloth. The second image is a photo of the violets in a pile on the dishcloth. The video shows the violets in a glass bowl. Water is poured over them from a pan, and they are gently pressed down and stirred into the water with a spoon. END ID]
also I may have accidentally made asmr. excuse the shitty lighting & the sound of my mom's hallmark movie running in the background.
I'll update with the finished product later when I have time to take a picture. I haven't tried it yet cause I don't have cheesecloth to filter out all the plant tidbits & also it smells like spinach but. it is Very Blue.
#(also it's a natural ph indicator so I can make it pinkish-purple or teal by adding lemon or baking soda respectively >:3)#violet tea#should I tag this cottagecore or smth? idk#we'll see if just having the word in that tag brings anyone in. the Tumblr search function is forever a mystery. as it should be.#ok lets see if the video posts#I have no idea if I did the image ids correctly.
4 notes
·
View notes
Text
Phenolphthalein is therefore a nearly perfect indicator for the titration of a weak acid by a strong base, where the pH at the equivalence point is on the basic side (figure 11.19).
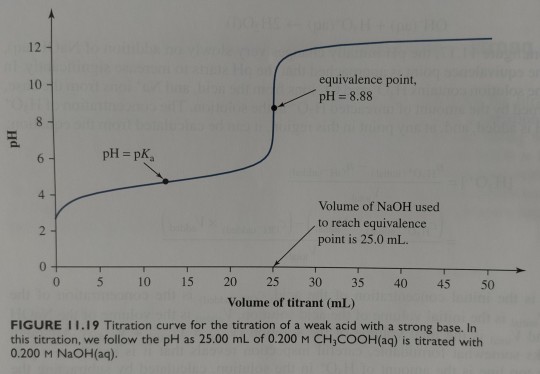
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#phenolphthalein#indicator#titration#acid#base#ph#basic#chemical reactions
0 notes
Text
if called alizarin red s why does it turn purple. alizarin purple s...
2 notes
·
View notes
Text
Soil test kits (figure 11.10) use a 'universal indicator', which is a mixture of indicators that enable estimation of pH over a wide range.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#colorimetric test#soil ph#ph test kit#universal indicator
0 notes
Text
Bcecf am special packaging instructions
cytosolic pH of Shop.
Learn about or buy BCECF AM from Sapphire Bioscience. A widely-used fluorescent indicator for estimating intracellular pH; amenable to either
With a pKa of 6.98, the cell-permeant, dual-excitation ratiometric pH indicator BCECF, AM is ideal for measuring changes in the cytosolic pH of most cells.
loaded into viable cells, such as Calcein-AM, BCECF-AM,. Carboxyfluorescein succinimidyl ester (CFSE), BCECF-AM Special packaging orange-brown solid.BCECF, AM. Recommended Use: For Research Use Only. Not for human or veterinary use. Check out our current Promotions
BCECF, AM ester is membrane-permeant and thus can be loaded into cells via incubation. This product is a mixture of three molecular species,
</p><br>https://mupotunefep.tumblr.com/post/692464908996083712/stuva-grundlig-drawer-instructions, https://mupotunefep.tumblr.com/post/692464908996083712/stuva-grundlig-drawer-instructions, https://mupotunefep.tumblr.com/post/692464908996083712/stuva-grundlig-drawer-instructions, https://mupotunefep.tumblr.com/post/692464780505726976/singer-117-manual, https://mupotunefep.tumblr.com/post/692464780505726976/singer-117-manual.
#http://vk.cc/c7jKeU#nofollow#<p> </p><p> </p><center>BCECF AM SPECIAL PACKAGING INSTRUCTIONS >> <strong><u><a href= rel= target#<br> calcein am#<br> bcecf excitation emission#<br> bcecf sigma#<br> bcecf-am sigma#<br> a rapid method for measuring intracellular ph using bcecf-am#<br> corona green#<br> b1150 thermo#<br>#<br> </p><p> </p><p> </p><p>With a pK a of ∼6.98#the cell-permeant#dual-excitation ratiometric pH indicator BCECF#AM is ideal for measuring changes in the
1 note
·
View note