#Dyes
Text

Kaleidoscopic Dye Mod rocks!! and their polished variations :3
#minecraft#minecraft mods#mineblr#modded minecraft#pixel art#mc#dyes#el and l's dye mod#kaleidoscopic#rocks#stones#minecraft modding#minecraft art
67 notes
·
View notes
Text




Italian Dyer's Notebook
Autograph manuscript, circa 1856-1866
This warped and worn nineteenth-century Italian manuscript appears to be a working manual and color inventory of a wool dyer in mid-nineteenth-century Italy. The handwritten entries are dated between 1856 and 1866, suggesting that the notebook was used and added to over a period of time. The work includes more than 500 numbered and itemized recipes for dyes. Recipes are illustrated with more than 800 wool and fabric samples adhered to the pages. The samples range in colors from shades of brown to vivid fuchsia, turquoise, and mustard. The samples include fabrics of wool, felt, and cotton, as well as raw wool and coils of yarn. Ingredients listed include mud, urine, arsenic, and vitriol. Pages 192-219 contain longer descriptions of dying processes, one attributed to Giacomo Udinese and another to Cesare Bizzi.
Check it out on our digital collections site.
#colors#colors in textiles#colorfastness#dyes#dyes and dyeing#textiles#wool#italian manuscripts#manuscripts#rare books#old books#rare book#dye samples#19th century#othmeralia
526 notes
·
View notes
Text

Making Twigs x Dye Mod compatibility! I need designs for the pots on the right, if you'd wanna drop a suggestion that would be very much appreciated!
166 notes
·
View notes
Note
Do you know much about dyeing with lichens? I don't know much about it myself but am curious on what the standpoint is from those who study them. Ethical harvesting has been a huge thing I have heard from dyers in what I have seen.
I do know a bit! I got really into collecting lichens for dying, but when it came to the actual dying part I kinda lost my hyperfocus. But everything I learned came from "Lichen Dyes: The New Source Book" by Karen Diadick Casselman, which has a great section on the ethics of harvesting. I try to only collect lichens for dying that:
I can recognize
I know are abundant
are detached from the substrate or are otherwise in danger of being destroyed
are in unprotected areas
I find plenty of material in roadside gutters, in parking lots, on walking trails and sidewalks, on broken or cut branches, at parks and just about every tiny green space around shops and office buildings, etc. etc. I would never advocate for large-scale or commercial harvesting or collection, the impact should be insignificant in the grand scheme of things, and is a great way to learn about and connect with your local lichen species.
115 notes
·
View notes
Text
Scientists have developed a water-soluble, non-toxic fluorescent spray that makes fingerprints visible in just a few seconds, making forensic investigations safer, easier and quicker.
Latent fingerprints (LFPs) are invisible prints formed by sweat or oil left on an object after it's been touched.
Traditional forensic methods for detecting fingerprints either use toxic powders that can harm DNA evidence, or environmentally damaging petrochemical solvents.
Continue Reading.
99 notes
·
View notes
Text

everything's in :3
special thanks to Eka for help getting the sheep working ^w^
217 notes
·
View notes
Text
Researchers at Imperial College London have genetically engineered bacteria to grow animal- and plastic-free leather that dyes itself.
In recent years, scientists and companies have started using microbes to grow sustainable textiles or to make dyes for industry -- but this is the first time bacteria have been engineered to produce a material and its own pigment simultaneously.
Synthetic chemical dyeing is one of the most environmentally toxic processes in fashion, and black dyes -- especially those used in colouring leather -- are particularly harmful. The researchers at Imperial set out to use biology to solve this.
In tackling the problem, the researchers say their self-dyeing vegan, plastic-free leather, which has been fashioned into shoe and wallet prototypes, represents a step forward in the quest for more sustainable fashion.
Read more.
39 notes
·
View notes
Text
Creating custom dyes can be so magical. ✨️
Hello all, I hope you're able to find some time to relax this weekend. :)
I'll be using this dye mix on a new creation for this upcoming Makers Monday, but wanted to share a little sneak peek into the process beforehand.
Wishing you all the best. 🧡
#magic#sparkles#dyes#art#crafting#a little sparkle for your Saturday#witch in the workshop#archer inventive
391 notes
·
View notes
Text

The last of the Bateman extended manifold twill. Linen warp and tabby, wool pattern Weft. Once washed I randomly applied dyes which was an experiment.



#handweaving#linenyarn#8shafts#slowcraft#woolyarn#handweaversofinstagram#louet david#handdyed#dyes#extended twill
32 notes
·
View notes
Text
Not qsmp, but I think being able to dye Minecraft books would go so hard.
Though imagine different qsmp members having different book colors as a like..way to say they wrote it.
Someone said the qsmp does have this but is it just different books or the ability to dye them. Cause I specifically mean able to dye them. And even so, (why I put non qsmp) I want this. Lmao
#is there a mod for this#if not can someone please make one#i need this#yeah anyway#qsmp#kinda#Minecraft modding#Minecraft#minecraft book#dyes
16 notes
·
View notes
Text


Weevil! This guy loves acorns.
#minecraft#minecraft mods#modded minecraft#mineblr#pixel art#mc#dyes#el and l's dye mod#weevil#bug#buggy#acorn weevil#beetle
5K notes
·
View notes
Text
Adventures in Plant Dyeing: Part 1 - Yarrow
Last week while at our monthly re-enactment group training meeting, my friend Jess who is a very accomplished dyer pointed out that there was yarrow growing in the field we were in. Naturally I had to pick some and came home with an armful to dye with the next day. Yarrow is recognisable by its small white flowers and feathery leaves as shown in the photo (not mine).

Yarrow produces a greenish-yellow to bright yellow colour depending on the mordants used and the particular harvest you use. I had several undyed or otherwise cream-coloured skeins of yarn I could use, and I chose 50g of a wool-nylon blend yarn. A quick google check suggested that nylon does indeed take dye, although not as well as natural fibres like wool. For fresh plants you're meant to use about a 1:1 ratio of plant matter to fibre, however after weighing it I realised I had 120g of yarrow. I decided to use it all anyway because I didn't want to have the remainder laying around without anything to use it for.

Firstly I mordanted the yarn using alum granules, I used about 8g for the 50g of fibre. For this I set some water on the stove in a large metal pot (called a dye pot) and dissolved the alum in a cup of hot water. I poured the dissolved alum in the pot and added the wet yarn, then kept it at roughly 80°C (180°F) for 45 minutes. I then removed it and left it in the sink until it was ready to go into the dye bath itself.
To prepare the dye bath, I chopped the yarrow into rough pieces, not too finely (although I found sources that both cut up the yarrow and left the plants whole) and placed them into the dye pot in fresh water. I brought it to boiling then simmered it with the lid on the pot for an hour. After this I added the skein of yarn without removing the yarrow and kept it simmering for another hour. It filled almost the whole house with a strong scent of plants which was rather unpleasant, so I recommend doing this with the windows open. Then I turned the heat off and left it to cool down until about 9pm, which was about 6 or 7 hours later.

I removed the yarn and rinsed it until the water ran off clear, then I hung it up to dry overnight. In the morning I wound it into a ball for use in tablet weaving and braiding.
Here's a before and after photo for comparison. Overall I'm quite happy with how it turned out, especially since I probably didn't pick the yarrow at the optimal time and the yarn was a nylon blend.


61 notes
·
View notes
Text
I've been meaning to make more posts about this, but there's so much i have to say/images to show that this will be a part 1 out of ??? and this one focuses mainly on the first batch of Purple Iris watercolors and the pH mystery they made me unravel through chaotically-organized researching.
Basically, I've been messing around with anthocyanins, a common class of plant pigments that are pH sensitive/can be used as a pH indicator. The first source I've tried has been purple irises, which i've only vaguely been familiar with in the past. The ones I picked were the ones that had begun to shrivel slightly, to the point where they were still a deep purple but picking them they would almost be leaking a purple liquid that stained my hands. I put them in a thing of hot tap water (not boiled, just the hot setting on the faucet), enough to cover the flowers, and let them steep. they began changing the color of the water almost immediately, with the fresher ones not losing their color as quickly as the ones that had begun to wilt on the plant. within 30 minutes i decided it was extracted enough.

This left a strong purple in the water, which i then poured off into three other containers, two of which i would alter the pH of.
The purple is due to delphinidin, a type of anthocyanidin that forms the building blocks of anthocyanins. Note i italicize the word anthocyanidin just so it's easier to tell apart the two.
there are anywhere from 16-31 anthocyanidins depending on what source you find, but they are basically the backbone structure of anthocyanins, of which there are over 600 something. The main thing that turns an anthocyanidin (aglycon) into an anthocyanin (glycoside form) is a sugar attached to it.
Realistically, that distinction isn't useful when simply extracting things from flowers in hot water, but i thought it was a fun fact to note. Anthocyanidins also come in handy for knowing what builds the anthocyanins in your flowers/plant part;
cyanidin (30%), delphinidin (22%), and pelargonidin (18%) make up the base for a good majority of all the anthocyanins in plants (~60% collectively),
peonidin, malvidin, and petunidin being runnerups (20% collectively)
the 20-something remaining anthocyanidins make up the rest
So basically, they all have slightly different colors that are pH reactive, and can provide anything from red to pink to orange to purple to blue. But, for our purposes, if you have a blue/purple flower, that likely means it has some amount of delphinidin-based anthocyanins in it! there can also be more than one anthocyanidin type present in the same plant.
Other well-known sources of anthocyanins are grape skins, red cabbage, red onions, butterfly pea tea, and purple violets. However, they're also very abundant in many many other plants, these are just the common ones i can think of that lots of people are probably familiar with to some degree.
Fun fact, grape skins are actually really well-studied as far as anthocyanins go (i believe they mainly have malvidin-based ones) because they're so important for the coloration of wine!
Anthocyanins as a whole are also studied as a natural source of food dyes, along with other flavonoids such as carotenoids.
As for why it turns colors, this is because of the way the anthocyanin changes structure in different pHs. The short answer is it turns red/pink in low pH (acidic) conditions, purple in slightly acidic/neutral conditions, and blue/green in slightly high pH conditions.
The long answer is something I'll explain in a moment, but for now here's the acid/base colors:


(on the left, i altered it with vinegar and it became a bright magenta color; on the right, i altered it with baking soda and it became a sea green/blue cyan that refused to show up accurately on camera). That's one thing I've noticed, and others have too, is that when working with pigments (especially natural ones) the color accuracy of the camera often just completely fails. there's only so many colors a digital camera can capture!
Here's a slightly more accurate color due to different lighting, note how it's more a malachite green than a pure blue. off the bat this was interesting becuase I wasn't expecting as green of a liquid as i got.

Anyways, the first thing i did with them was use them as-is, no alterations past the addition of the respective vinegar and baking soda. I painted with them just as one would paint with watercolors, and interestingly enough, when i put them onto paper, they began to change from their pink/purple/malachite colors to a teal/indigo/emerald set instead.
This seems to be the result of something in the paper itself, likely calcium carbonate (which i only recently learned is added to "buffer" paper against acidic substances; the cellulose in paper is more stable long term when there's no acid present, and the calcium carbonate neutralizes any acids applied to a degree).
It's still interesting that even though the acids are neutralized, they give a unique color when compared to the basic paint.
I also tried soaking some of the same paper in vinegar water, which got rid of that buffer and let me paint with the pinks intact, but that's for another post.
Also, note that i said "neutral" for the middle, this is just what i wrote down for the tap water sample; in actuality the tap water is actually a bit closer to pH 6 instead of a true neutral 7, which i only found out after i had gotten this far. So whenever i say "neutral," i mean "tap water that's slightly acidic"
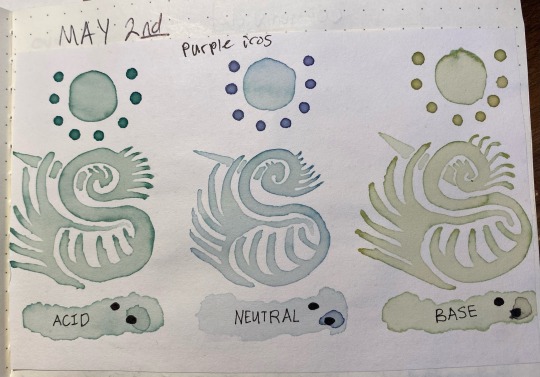
Here's something interesting that happened, though. Overnight i left the jars on my desk, and while the acid and neutral colors were the same when I came back ~24 hours later, the basic had degraded into a murky brown. this was interesting since that meant the instability was pH-dependent.
So, i made another color swatch with the acid/neutral/base, and used that as a comparison to look at how it had changed. Surprisingly, it painted out a yellowy-green instead of a murky gray-brown
here's the murky water that the once-malachite-green turned into:

I also poured off a bit into another container, and shifted it back into a low pH with a bit of vinegar to see if it would still change color. Surprisingly, it turned a slight pink, like pink lemonade, which means there were still anthocyanins in there but they were likely a lot less concentrated than they used to be.
Here's the pink, with a few leftover bubbles from the baking soda/vinegar reaction:

Here's the results of painting with these:
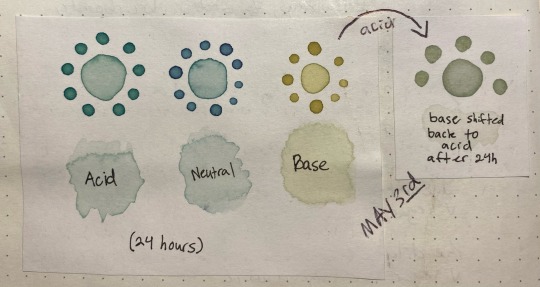
the acid was basically unchanged after sitting in a jar for 24 hours, the neutral had lost a bit of its purple color but was still about the same, and the base was now a lot yellower/tanner with a bit of green still showing through. The shifted sample was a pale stormy gray that ended up taking on a green color as it dried, as though following the trend of pink shifting to a bluer color but on a much more muted scale.
Now obviously, i wanted to figure out what caused this, so i dug around both on wikipedia and other sites but found myself eventually reading into scientific papers on the topic, at which point it became very clear that i would need to learn like, organic chemistry and such to be able to say for sure what was happening.
I did eventually manage to figure out a few things despite the dense terminology; for one thing, anthocyanins are more unstable than other plant pigments such as carotenoids. there are plenty of things that can affect their stability, including the pH of the substance they're stored in. Any higher than pH 7 (basic pHs) and theyll begin to degrade. This explains why the high pH sample lost its blue/green color, and why there was very little left to be shifted back to a pink color.
I also found out that the pH color shift isn't as simple as it seemed. Rather, there are multiple chemical forms of anthocyanins.
At the lowest pHs, basically all of them are in the "flavylium cation" state, which basically means it's positively charged and this is what gives a red color
still at a low pH (2-4), there's anothe chemical form that appears, the "quinoidal" structure that gives a blue color. Note that the red cation is still present, just no longer the only form
the more the pH rises, the more forms start to coexist, with some of those forms being colorless (one of which is a "colorless carbinol"
so, between 4 and 6 there are the cations (red) quinoidal (blue) carbinol (colorless) and something called a chalcone that gives a pale yellow
and then past that, I'm unsure, but of course that's around when the anthocyanins begin to degrade
There are also a lot more than these that i've encountered in various contexts but these seem to be the basic ones.
Do note that i do not fully understand these terms (flavylium, quinoidal, carbinol, chalcone, etc.) and have only recently begun to actually try to learn what they mean and the context surrounding them as i only had a class of basic high school chemistry under my belt prior to this. The main paper i combed over to try to find info on it seems to be behind a paywall but the DOI is:
doi.org/10.1016/j.foodchem.2008.09.001
for anyone curious and able to access it, whether through legit means or what have you.
That being said, to me, the takeaway here seems to be that there's a yellow form that appears around the time that other color forms begin to disappear, and as those degrade it makes sense that the resulting degraded forms also contribute to a murky color. This helps explain why it changed color in the jar and also retained a bit of yellow and green.
This also explains why the blue form seems to also be slightly green, it's got the blue quinoidal chemical form as well as the yellow chalcones.
There are also interesting things of note that I will get into at a later date, such as the texture/reflectivity of the way it dries, the differences in extraction ease between this and purple violets, the addition of a genuinely neutral/pH 7 sample later, a sample from a plant that doesnt seem to have delphinidins, and sample the seems to genuinely sparkle??? Much more of interest to come soon!
#art#traditional art#anthocyanins#watercolors#purple irises#painting#science#natural dyes#pigments#dyes#pH indicators
61 notes
·
View notes
Text

I want sun-block for this one.
Another sun-face but this time creepy and Riddler-esque. Any ideas on the riddle? (Novelty prize is expired)
Color trade journal and textile chemist, May 1925.
32 notes
·
View notes
Text




Testing out some new dyes.
I didn't intend for the second egg to make me think of icing and gingerbread but it kind of does, lol
8 notes
·
View notes
Text
Tips for Lichen Foraging
If you enjoy using lichens for dying, crafting, decoration, perfumes, etc., or you think you want to start, here are my tips for going out there and collecting these funky little friends in a safe manner.
1. ALWAYS harvest ethically! This means collecting lichens that are not longer in situations where they can thrive (detached from their substrate, in roadways/walkways, are likely to be destroyed, etc.), only collection lichens that are abundant and thriving, and leaving 9/10 of the lichens in any given area undisturbed.
2. Location: I find urban locations to be ideal for harvesting due to the large amount of material that can be scavenged from debris piles, roadways, walkways, woodpiles, and other areas that are not ideal for lichen growth, and places where the lichen and/or its substrate are going to be destroyed/thrown away. I often scavenge around areas where trees are being trimmed back/removed ,but do this safely, legally (without trespassing) and if possible, with consent to do so. I happen to live right by a few parks subject to regular tree trimming, as well as a commercial conifer wood that is regularly logged. But honestly, the best locations I have found? Parking lots. Yep, those trees in the grassy median of parking lots drop a lot of lichen, and the smooth roadway surface and gutters make the lichens there really easy to see.
3. Season: Winter is the best time of year to look for lichens! There is less foliage to pick through, and the bright color of the lichens stands out better against snow/dead leaves.
4. Identification: Make sure you can identify every species you collect en masse. There are lots of species that are abundant in urban areas that are easy to identify, and I recommend you start with those. Also, look online for local field guides for lichens in your region--you'll be amazed what you can find! And if you need help with identification, you can post pictures to sites like iNaturalist and GBIF and Mushroomobserver, where the community can help with ID.
5. Check for critters! Many lichens and their substrates harbor invertebrates, so try to disturb as little as possible when foraging, and leave behind as much dirt/rock/branches/bark/moss/etc. as you can. When I get my lichens home, I "process" them as soon as possible, checking them for bugs and removing other biological material so that I can return them to the outside right away. Better for you, and better for the environment.
6. Lichen foraging is a hobby, not a business. Lichens are slow growing, sensitive, and in peril in many regions. Relying on lichens for a business can often lead to overexploitation. So have fun, be safe, and treat lichens and the natural world with respect and kindness.










I am sure I am forgetting something, but I am happy to try and answer any questions anyone might have about lichen foraging/harvesting. For more tips on ethical and safe harvesting, I recommend Lichen Dyes: The New Source Book by Karen Diadick Casselman, which is where most of these tips came from.
#lichen#lichens#foraging#forager#urban foraging#natural dying#dyes#the cat's name is Aldi#he is bad at lichen foraging#but a very good boy otherwise
72 notes
·
View notes