#bromine
Text
Bromine makes me unreasonably nervous.
That being said there aren't many MSDS that say "inhalation will cause death"

224 notes
·
View notes
Text
Round 2 - Matchup 17


Bromine 35 (Br) - Red-brown liquid that evaporates into a red-brown gas. It's a halogen so it'll basically react with anything, usually violently.
vs
Bismuth 83 (Bi) - Rainbows and right angled crystal structures. It's radioactive but its half life is longer than the estimated age of the universe. Often used to replace lead as a less toxic alternative.
94 notes
·
View notes
Text


source<3
31 notes
·
View notes
Note
my ABSOLUTE FAVORITE ELEMENT is Bromine! So I was wondering… if you know anything cool about it?

(In other words, this is me begging you for an info dump on Bromine)
🧪 BROMINE 🧪
Element No. 35, bromine, is a fairly abundant element but has a rare property: it is the only nonmetal to exist in liquid form at room temperature & one of only two elements (the other being mercury) that is liquid at room temperature AND pressure.
It has a brownish-red colour with a bleach-like odor & it dissolves in water.
Bromine is found naturally in the earth’s crust & in seawater in various chemical forms (actually in any natural water that has salt in it)
Products containing bromine are used in agriculture & sanitation & as fire retardants (chemicals that help prevent things from catching fire).
Some bromine-containing compounds were historically used as sedatives.
✨️ Now, some facts ✨️
The name bromine comes from the Greek word "bromos" for stench
Bromine is one of seven natural diatomic elements, a molecule made of two identical atoms: Br2
Bromine is a halogen: such elements (fluorine, chlorine, bromine, iodine & astatine) are never found alone in nature & produce salts
Bromine is very harmful to the atmosphere. According to Chemicool, bromine atoms are 40 to 100 times more destructive in the ozone layer than chlorine atoms!
It is also very damaging for living organisms: It is corrosive to human tissue in its liquid state & it irritates eyes & the throat & is highly toxic when inhaled in a vapor state. Bromine damages many major organs, including the liver, kidneys, lungs & stomach, and, in some cases, can cause cancer.
In ancient times, bromine played a role in the colouring of clothing dyes, specifically Tyrian purple, also known as dibromoindigo (6’6 C16H8Br2N2O2). Spiny-dye murex snails retrieved bromine from the ocean & bound it to the indigo molecule on land.

I do hope you learned something new! Please let me know & I thank you for the opportunity to infodump! /g
#thyrian purple T^T#i love to infodump#i LOVE chemistry /lie#donnie speaks#donatello infodumps#donnies exceptional mind#turtle net#science facts#bromine#periodic table of elements#autistic infodumping#infodump#rottmnt autistic donnie
13 notes
·
View notes
Text

Br(Bromine)
No. 35
Group 17
He | Him
——-

#ATOMS#Br#bromine#ATOMS story#ATOMS comics#periodic table#elements#chemistry#science#character#oc#illustration#digital painting#procreate#art#artist on tumblr#web comic#webcomic#web comics#webtoon#chibi art#character design
32 notes
·
View notes
Text

#periodic table poll#periodic table of elements#einsteinium#bromine#polls#bracket poll#chemistry#science#round 2#branch a
21 notes
·
View notes
Text
HALOGENS:
ROUND 1 POLL 2


BROMINE:
One of only two elements that are a liquid at room temperature
The only nonmetal element that is a liquid at room temperature
Highly hazardous and can leave scars if spilled on skin. Despite this, it is sometimes added to vegetable oil
IODINE:
used to make the first photographs
Radioactive iodine can cure thyroid cancer
9 notes
·
View notes
Text
Round 9 - Halogens & Noble Gases
#polls#periodic table#chemistry#fluorine#chlorine#bromine#iodine#astatine#tennessine#neon#argon#krypton#xenon#radon#oganesson
3 notes
·
View notes
Text
Table 20.2 lists some accurate masses for the elements commonly encountered in organic chemistry, along with their natural abundances. (...) You may have noticed that, of the elements listed in table 20.2, many had one isotope with a natural abundance of more than 99%.
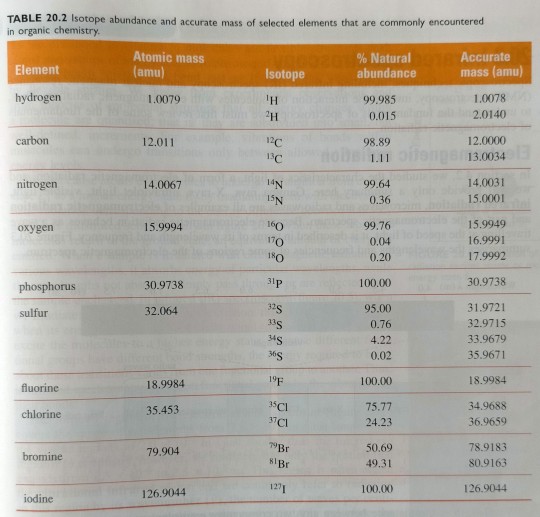
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#organic chemistry#isotope#hydrogen#carbon#nitrogen#oxygen#phosphorus#sulfur#fluorine#chlorine#bromine#iodine
2 notes
·
View notes
Text

BROMINE
i love hex hes so silly
43 notes
·
View notes
Text
The electrophile is a halonium ion, Cl+ or Br+, formed by treating Cl2 or Br2 with AlCl3 or FeCl3:
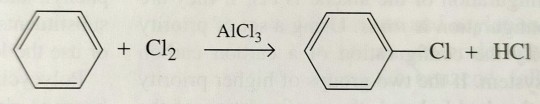
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#chlorination#bromination#electrophile#halonium#ion#cation#chlorine#bromine#aluminum chloride#iron chloride#aluminum#iron#chemical reactions
2 notes
·
View notes
Text
The addition of bromine and chlorine to a cycloalkane gives a trans-dihalocycloalkane. For example, the addition of bromine to cyclohexene gives trans-1,2-dibromocyclohexane; the cis isomer is not formed.
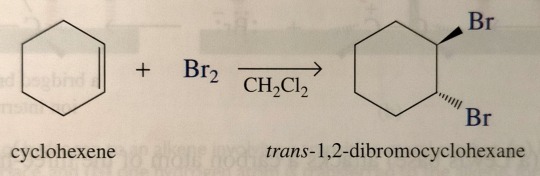
Addition of bromine to a cycloalkene is highly stereospecific; the halogen atoms always add trans to each other.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#bromine#chlorine#cycloalkene#trans#cyclohexene#chemical reactions#dibromocyclohexane#halogen#alkene#stereospecific#isomer
2 notes
·
View notes
Text
Round 1 - Part 2 - Matchup 14
Bromine vs Berkelium


39 notes
·
View notes
Text

#monosacharides#hydrolysed#aldehydes#ketones#chemistry#solutions#oxidation#glucose#bromine#water#glutamic acid#anomers#hemiacetal#fructose
3 notes
·
View notes
Text
i think more writers/artists should incorporate bromine-based powers into their works.
its one of the only two liquid elements at room temperature, it kinda looks like orange blood, it turns into smoke on its own, its extremely toxic even at the smallest dose and highly reactive.
its perfect for a creepy poison-based or elemental villain
also its just so damn cool
#bromine#am i really the only one with a character based on bromine?#their name is Brom and i love them#bromine god/elemental who is just a droopy little slug guy that kills everything by accident but doesnt really care#Brom my beloved#iggy rambles
3 notes
·
View notes
Note
Can I offer you a drink? It’s not poisoned or anything, it’s never been poisoned. Ridiculous thing to even wonder, really. As if I would ever poison someone. Anyway, here’s a bromine on the house.
Some bromine-containing compounds were historically used as sedatives (drugs that can make people calm or sleepy). However, these drugs are for the most part no longer found on the market in the United States.
The seriousness of poisoning caused by bromine depends on the amount, route, and length of time of exposure, as well as the age and preexisting medical condition of the person exposed.
imma pass
2 notes
·
View notes