#chemistryblr
Text
NOW FOR ROUND 2
THE MOST FUCKABLE GROUP OF THE D-BLOCK
once again, reblog please, large sample size yada yada
#science#science side of tumblr#chemistry#nerdpana#poll#polls#chemistree#periodic table#periodic table poll#most fuckable element of the periodic table#periodic properties#fun#shitpolling#shitposting#desiblr#scienceblr#chemistryblr#desi shitposting#studyblr#studentblr#nerdblr
83 notes
·
View notes
Text
(I'm doing practise for my chemistry exam and reading aloud the practise questions, dad enters the room.)
My mum to my dad, about me: They're reading out their chemistry problems as they go along
My dad: Oh, I'm so sorry
1 note
·
View note
Text


saturday night
#studyblr#dark academia#chemistry#dark acadamia aesthetic#physics#chemistryblr#math#mathblr#physicsblr#astronomy#astrophysics#brown aesthetic#aestethic#studygram#langblr#stars#space science#nasa#spacex#science#quantum physics#computer science#study motivation#studying#constellations#solar system#intj#intp#lesbian#lgbtq
222 notes
·
View notes
Text
what
what if i told you
that here in Russian schools we use the periodic table like this?...
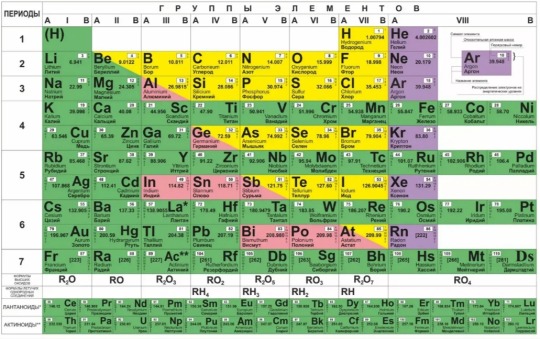
#chemistry#chemistryblr#studyblr#yeah russians are a bit weird#telling you this as a russian actually#this is the periodic table noble gas is stable...#also idk if that changes in university#like i'm freshman so nobody cares#for now at least
3 notes
·
View notes
Text
Snippets of some notes


Exams are soon so I'm spending my time revising... these can all be found on my dropbox links.
#Original#knockingonglass studies#Study#studying#exams#notes#exam notes#science#biology#chemistry#A-level#A-levels#A-level chemistry#A-level biology#Studyblr#studyblr#study blog#scienceblr#biologyblr#chemistryblr#highschool#sixth form#notability#iPad
31 notes
·
View notes
Text
Remembering when my chemistry class called viscous liquids the thicc-quids and made the teacher, who put that as a choice on the test, a correct answer since almost everyone chose thicc-quid thinking he would do that since he would pull shit like that. Really miss that teacher.
#school#chemistry#science#I WILL HAVE IT SO SCIENCE BLOGS SEE THIS POST AND BE DISAPPOINTED IN MY CLASS#story time#scienceblr#chemistryblr
11 notes
·
View notes
Text
A-level bio, chem, psychology and math peeps
I need your help
You got any tips? I’m about to start a-levels and although I’ve done a lot of prep, I know it’s a MASSIVE step up from GCSE, so I need some guidance.
I’m wanting to study Medicine at uni
THANK YOU ALL
#studying#biology#chemistry#psychology#maths#a levels#as levels#studyblr#scienceblr#medblr#mathsblr#biologyblr#chemistryblr#psychologyblr#studying tips#revision
11 notes
·
View notes
Text
hi there analytical chemistry side of tumblr! (is that even a thing ? hell idk) this might kinda be a bottle in the sea but imma try it anyway : so i've been using a hplc system, although i'm everything but an expert about it, and i'm afraid that the column i'm using might have been retrieved and stored for 1+ month without any rinsing (no proof tho, i'm not the one who got it out and assumed back then that proper rinsing had been made). i've had a few issues with the results i got yesterday (peaks missing, wrong retention times, etc) and first thought there was a leak, but apparently not, and i'm now suspecting the column/the system
my column is a rt c18 one, advised to be rinsed with 100% acn before storage. i've been told rn to purge the whole system with milliQ water and then flow the column for 30 min with pure ACN but in case this wouldn't work: have you any tips for what might need to be done to retrieve/regenerate a column that hasn't been rinsed before long storage? the despair is so strong with this one rn
thank you for anyone who might have an idea! you'd be saving a poor phd student's life
7 notes
·
View notes
Text

☆Summer time in the laboratory!
#science#chemistry#chemist#chem#chemistryblr#scientists#laboratory#lab#scientist#laboratory life#reblog#chemical#tumblr
12 notes
·
View notes
Photo





Ionic equilibrium notes.
Find more at @purhplestudies on instagram
#notes#chemistry#a levels#chem#chemistryblr#chemistry notes#summary#studyblr#chemblr#ionic equilibrium#study#studyspo#chem notes#zebra mildliners#mildliner zebra#mildliner#mildliners#purhplestudies
30 notes
·
View notes
Photo




Still upset with my lighting, my house is so dark. I may have to experiment with going outside of my room 😨 Anyway, here's some notes of periodicity that I hope are readable. I remember this as being one of my least favourite AS topics, but it doesn't seem too bad. My chemistry mock is in a week so gotta get this stuff down.
#chemicalgladiator#periodicity#periodic table#chemistry#chem#chemblr#chemistryblr#chemistry blog#a level chemistry#as level chemistry#science#ocr#studyblr#new studyblr#study#studying#study notes#notes#school#sixth form#a levels#as level#learning#student#education#revision
380 notes
·
View notes
Text
Structural Isomerism
In one boring history lesson, you and your friend (who both love chemistry) are doodling displayed formulas in the back of your textbook. You both decide to draw C5H12 - however, when you come to name what you’ve drawn, your friend has something completely different. You know what you’ve drawn is pentane and your friend knows what they’ve drawn is 2,3-dimethylpropane. So which one is C5H12?
The answer is both! What you and your friend have hypothetically drawn are structural isomers of C5H12 (another is 2-methylbutane). These are compounds which have the same molecular formula but different structural formulas.
Isomers are two or more compounds with the same formula but a different arrangement of atoms in the molecule and often different properties.
There are several different kinds of structural isomers: chain, positional and functional group.
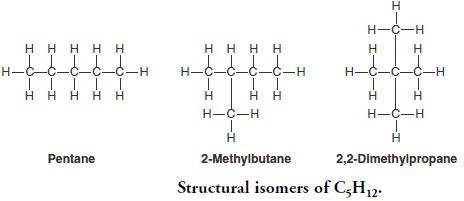
Chain isomerism happens when there is more than one way of arranging carbon atoms in the longest chain. If we continue with the example C5H12, it exists as the three chain isomers shown above. Chain isomers have similar chemical properties but different physical properties because more branched isomers have weaker Van der Waals and therefore lower boiling points.
Positional isomers have the same carbon chain and the same functional group but it is attached at different points along the chain.

This is a halogenoalkane. The locant “1″ describes where the chlorine is on the chain. For more on naming organic compounds, check out my nomenclature post.
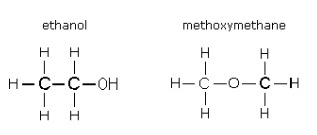
The final type of isomer you need to know is a functional group isomer. This is a compound with the same molecular formula but a different functional group. For example, C2H6O could be ethanol or methoxymethane.
And surprisingly, that is all you need to know for the AS exam. There are also things called stereoisomers but those will be covered next year. Just make sure you know how to name and draw the three different kinds of structural isomers for the exam. Practice makes perfect!
SUMMARY
Structural isomers are compounds which have the same molecular formula but different structural formulas.
Isomers are two or more compounds with the same formula but a different arrangement of atoms in the molecule and often different properties.
There are several different kinds of structural isomers: chain, positional and functional group.
Chain isomerism happens when there is more than one way of arranging carbon atoms in the longest chain. Chain isomers have similar chemical properties but different physical properties because more branched isomers have weaker Van der Waals and therefore lower boiling points.
Positional isomers have the same carbon chain and the same functional group but it is attached at different points along the chain.
A functional group isomer is a compound with the same molecular formula but a different functional group.
Happy studying!
#a level#a level chemistry#a level chem#isomers#functional groups#carbon#halogen#alkanes#formula#chemistry#chemistryblr#chemblr#studyblr#scienceblr#sciblr#sci#science#a level science#as level#aqa#aqa chemistry#as level chemistry#as level science#chemistryblog#free learning#learning#education#study#study help#help
118 notes
·
View notes
Photo

so I finally got round to writing out my study schedule and this is it atm!
#studyspiration#study#study motivation#study notes#studyblr#muji stationary#muji pens#muji#englishblr#english#mathblr#science#scienceblr#physicsblr#physics#biologyblr#biology#chemistryblr#chemistry
81 notes
·
View notes
Text
The Junior Year of College Hustle
2 am sleep
7 am jog
9 am - 12 pm lab
12 pm - 1 pm lecture
1 pm - 1:15 pm advisor meeting
1:20 pm - 1:50 pm study lecture
1:55 pm - 3 pm work on presentation
3:10 pm - 3:30 pm other advisor meeting
3:30 pm - 4 pm work on presentation
4 pm - 5:15 pm give presentation/lecture
5:30 pm - 7 pm study lecture
7 pm - 8 pm work on assignment for Wednesday night class
8:00 pm - 10 pm study for exam
10 pm - 1 am shower and then sleep
#im so tired#organic chemistryblr#chemistryblr#chemblr#organic chemistry#orgoblr#studyblr#3rd year#uni
4 notes
·
View notes
Photo


21.09.2017 // I've spent morning learning for my chemistry technology class which is tomorrow
4 notes
·
View notes
Text

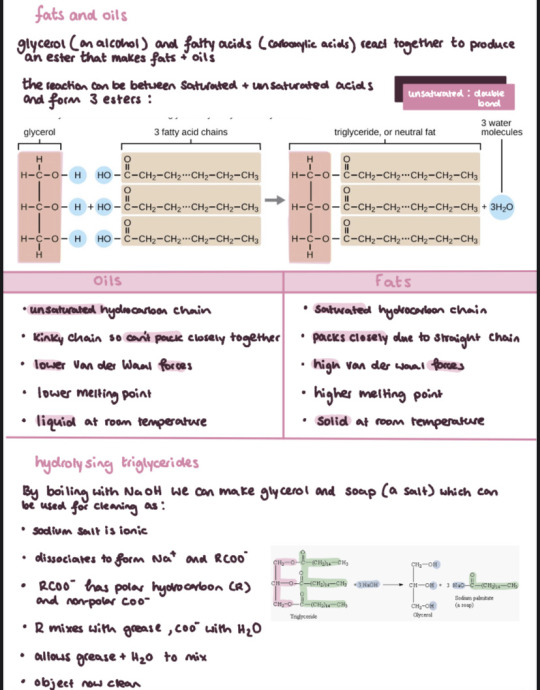
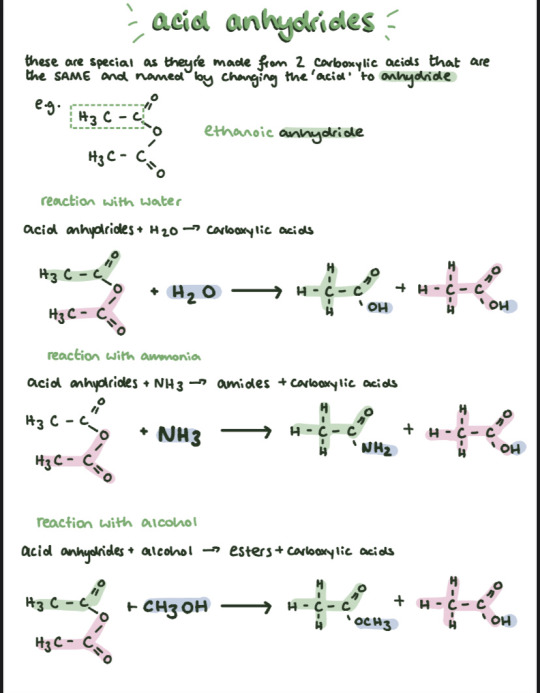
More note snippets cause I kinda enjoyed this chapter . Don't know why my teacher over complicated organic for us but it's actually not as terrible as I first thought!
(again all found on my Dropbox links which I'll keep updating as soon as I stop procrastinating haha!)
#knockingonglass studies#notes#notability#study aesthetic#aesthetic#study#studyblr#scienceblr#science#chemistry#chemistryblr#alevel chemistry#studying#exams#examstress#exam notes#exam#alevel#sixth form
14 notes
·
View notes